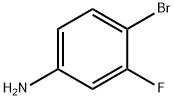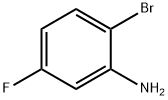
2-Bromo-5-fluoroaniline synthesis
- Product Name:2-Bromo-5-fluoroaniline
- CAS Number:1003-99-2
- Molecular formula:C6H5BrFN
- Molecular Weight:190.01
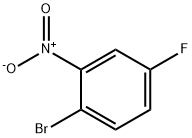
446-09-3
245 suppliers
$7.00/5g

1003-99-2
307 suppliers
$6.00/5g
Yield:1003-99-2 100%
Reaction Conditions:
Stage #1:2-bromo-5-fluoronitrobenzene with iron;acetic acid in ethanol at 20; for 2.08333 h;Heating / reflux;
Stage #2: with sodium hydroxide in diethyl ether;water
Steps:
2-Bromo-5-fluorobenzenamine; To a solution of 1-bromo-4-fluoro-2-nitrobenzene (5.0 g, 22.7 mmol) in HOAc/EtOH (20 mL/20 mL) was added iron powder in one portion at room temperature. The mixture was bubbled with N2 for 5 min, and then refluxed for 2 hrs. Partial of the solvents were removed on rotary vacuum, then the residue was partitioned between aqueous NaOH (10N, 200 mL) and Et2O (200mL). After separation, the organic phase was washed with H2O(50 mL), brine (50 mL), dried on MgSO4, and concentrated on rotary vacuum to afford the expected product, as an light tan oil (quantitative yield), which was pure enough to be used in next step without further purification; 1H NMR (400 MHz, CDCl3) δ ppm 4.15 (s, 1 H), 6.34 (td, J=8.44, 2.77 Hz, 1 H) ,6.46 (dd, J=10.20, 2.90 Hz, 1 H), 7.31 (dd, J=8.81, 5.79 Hz, 1 H); Mass spec. 189.94 (MH+), Calc. for C6H5BrFN 188.96.
References:
Bristol-Myers Squibb Company US2007/259850, 2007, A1 Location in patent:Page/Page column 95-96
909274-50-6
1 suppliers
inquiry

1003-99-2
307 suppliers
$6.00/5g
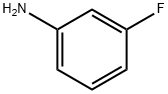
372-19-0
322 suppliers
$10.00/1g

1003-99-2
307 suppliers
$6.00/5g
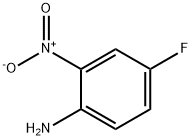
364-78-3
277 suppliers
$7.00/5g

1003-99-2
307 suppliers
$6.00/5g