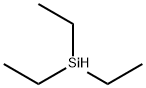
Triethylsilane synthesis
- Product Name:Triethylsilane
- CAS Number:617-86-7
- Molecular formula:C6H16Si
- Molecular Weight:116.28
To a 500ml reaction flask, 20g (0.5mol) of 60% sodium hydride and 80g of anhydrous tetrahydrofuran were added. Stirring and cooling to 0-10 ℃ under the protection of nitrogen, the mixture was then maintained under nitrogen protection, stirred, and the liquid temperature at 0-10 ℃, and dropwise added 57.2g (0.55mol) of trimethyl borate. After the dropwise addition, the temperature was kept at 0-10 ℃ and stirred for 1 hour.Maintaining the nitrogen protection, stirring and liquid temperature of the reaction liquid at 0-10 ℃, and then dropwise adding 67.7g (0.45mol) of triethylchlorosilane. After the dropwise addition, continuously keep the temperature at 0-10 ℃ and stir for 1 hour. The reaction solution was then filtered at room temperature. Rectifying the filtrate at normal pressure and collecting fractions with the boiling range of 109-110 ℃ as triethylsilane to obtain 46.1g of triethylsilane, which yields 88.2%. 45-64 ℃: a mixed solution of trimethyl borate and tetrahydrofuran; 64-109 ℃:a mixture of tetrahydrofuran and a small triethylsilane.
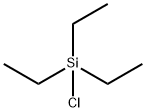
994-30-9
433 suppliers
$12.00/5g

617-86-7
533 suppliers
$9.00/5g
Yield:617-86-7 88%
Reaction Conditions:
with sodium tetrahydroborate in acetonitrile at 20; for 0.25 h;Inert atmosphere;
Steps:
2.2-3 Synthesis of example 3
After replacing the interior of a 7 mL test tube equipped with a stirring bar with nitrogen gas,Under nitrogen flow,In this test tube,0.5 mL of acetonitrile as a reaction solvent,As a reducing agent, sodium borohydride (NaBH 4, ReagentPlus (registered trademark), manufactured by Sigma-Aldrich Japan Co.,Purity 99%) 37.8 mg (1.0 mmol)To prepare a suspension.83.9 μL (0.5 mmol) of triethylchlorosilane (Et 3 SiCl) was added dropwise to this suspension using a microsyringe,The reaction was allowed to proceed for 15 minutes while stirring at room temperature.After the reaction, 18.6 mg (0.1 mmol) of ferrocene was added as a reference substance for calculating the NMR yield.A part of the obtained mixed solution was dissolved in heavy chloroform (CDCl 3)As a result of 1 H NMR measurement, it was found that triethylsilane (Et 3 SiH 4) was produced with an NMR yield of 88% (reference material: ferrocene).
References:
JP2017/160160,2017,A Location in patent:Paragraph 0039; 0041
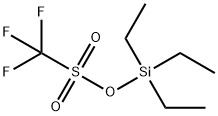
79271-56-0
244 suppliers
$12.00/1g

617-86-7
533 suppliers
$9.00/5g
67-56-1
764 suppliers
$9.00/25ml

617-86-7
533 suppliers
$9.00/5g
2117-34-2
34 suppliers
$3137.55/5GT

617-86-7
533 suppliers
$9.00/5g
![Benzene, [(triethylsilyl)seleno]-](/CAS/20210305/GIF/76358-43-5.gif)
76358-43-5
0 suppliers
inquiry

617-86-7
533 suppliers
$9.00/5g