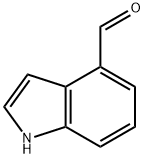
Indole-4-carboxaldehyde synthesis
- Product Name:Indole-4-carboxaldehyde
- CAS Number:1074-86-8
- Molecular formula:C9H7NO
- Molecular Weight:145.16
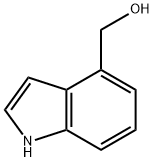
1074-85-7
100 suppliers
$27.00/250mg
114615-82-6
217 suppliers
$8.00/100mg

1074-86-8
298 suppliers
$10.00/1g
Yield:1074-86-8 80%
Reaction Conditions:
with 4-methylmorpholine N-oxide;silica gel in dichloromethane
Steps:
38.B Example 38
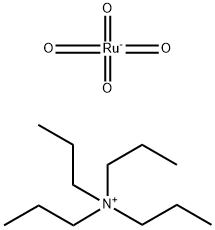
Step B: Tetrapropylammonium perruthenate (0.3 g, 0.85 mmol) was added in portions to a mixture of alcohol product from Step A (2.5 g, 17 mmol), N-methylmorpholine N-oxide (3.0 g, 25 mmol) and 4 A molecular sieves (3.0 g) in anhydrous methylene chloride (30 mL) at room temperature. The mixture was stirred at room temperature under nitrogen for 1 h and then filtered. The filtrate was concentrated in vacuo, and the residue was purified by chromatography (SiO2, CH2Cl2) to provide indole-4-aldehyde as a white powder (2.0 g, 80%): 1H NMR (300 MHz, CDCl3) δ 10.2 (s, 1H), 8.52 (br s, 1H), 7.64-7.69 (m, 2H), 7.31-7.44 (m, 3H); CI MS m/z=146 [C9H7NO+H]+.
References:
Beck, James P.;Pechulis, Anthony D.;Harms, Arthur E. US2002/91134, 2002, A1

1074-85-7
100 suppliers
$27.00/250mg

1074-86-8
298 suppliers
$10.00/1g
16136-52-0
257 suppliers
$10.00/1g

1074-86-8
298 suppliers
$10.00/1g
39830-66-5
306 suppliers
$14.00/1g

1074-86-8
298 suppliers
$10.00/1g
amine](/CAS/20200331/GIF/3468-22-2.gif)
3468-22-2
7 suppliers
inquiry

1074-86-8
298 suppliers
$10.00/1g