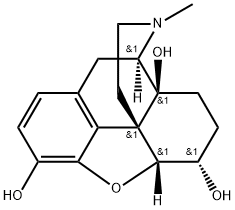
hydromorphinol synthesis
- Product Name:hydromorphinol
- CAS Number:2183-56-4
- Molecular formula:C17H21NO4
- Molecular Weight:303.36
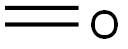
50-00-0
867 suppliers
$10.00/25g
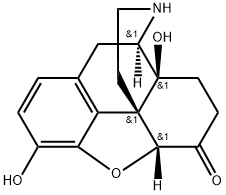
33522-95-1
87 suppliers
$60.60/013-1ml

2183-56-4
1 suppliers
$95.00/1mg
Yield: 92%
Reaction Conditions:
Stage #1:formaldehyd;noroxymorphone in acetonitrile at 20; for 16 h;
Stage #2: with formic acid;triethylamine in acetonitrile at 20; for 24.5 h;Product distribution / selectivity;
Steps:
2
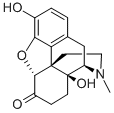
Example 2: Synthesis of 6-α-Oxymorphol[0076] Noroxymorphone (1.20 g, 4.17 mmole) was added to acetonitrile (5.0 mL).Paraformaldehyde (0.25 g, 8.33 mmol) was then added and the slurry was stirred for 16 hours at room temperature. At that time, a 5 to 2 mixture of 98% formic acid/ triethylamine [prepared by adding 98% formic acid (2.40 g, 52.1 mmol, 1.97 mL) to triethylamine (2.11 g, 20.8 mmol, 2.91 mL) in 5.0 mL of acetonitrile) was added. Dichloro(p- cymene)ruthenium (II) dimer (12.0 mg) was added followed by the addition of (1S, 2S)-(+)-N-tosyl-diphenylethylene diamine (15 mg). The reaction was purged by nitrogen gas (argon) for 30 minutes. After the nitrogen purge, a flow of nitrogen was allowed to pass over the reaction. The reaction was stirred for 24 hours at room temperature. HPLC analysis indicated that the reaction was complete (6-α-Oxymorphol: 98.9%, 6-β-Oxymorphol: 0.9%). The mixture was then evaporated to a thick oil. 5.0 mL of acetonitrile was then added and stirred at room temperature for 6 hours where a precipitate formed. The precipitate was removed by filtration and washed with 2.0 mL cold (5°C) acetonitrile. The precipitate was dried yielding the product (1.15 g, 92% yield).
References:
MALLINCKRODT INC. WO2008/137672, 2008, A1 Location in patent:Page/Page column 23
76-41-5
2 suppliers
$16.00/1mg

2183-56-4
1 suppliers
$95.00/1mg
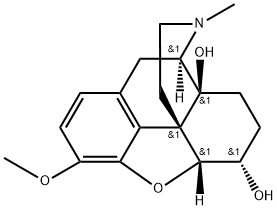
7183-69-9
19 suppliers
$65.00/1mg

2183-56-4
1 suppliers
$95.00/1mg
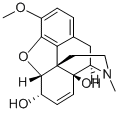
4829-46-3
15 suppliers
inquiry

2183-56-4
1 suppliers
$95.00/1mg