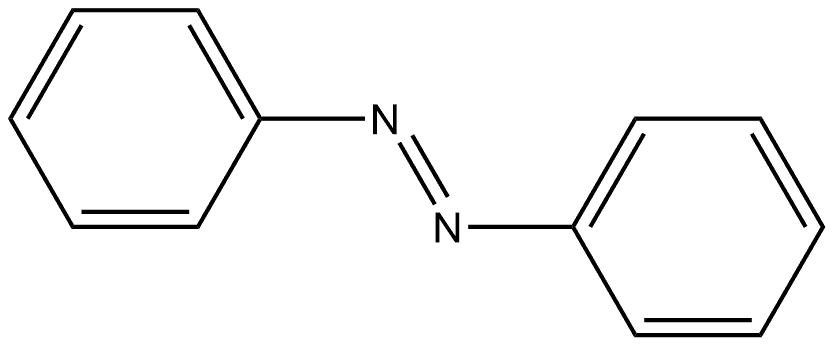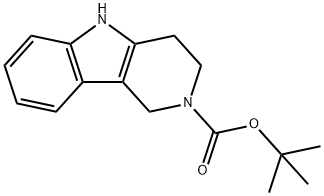
tert-Butyl 1,3,4,5-tetrahydro-2H-pyrido-[4,3-b]indole-2-carboxylate synthesis
- Product Name:tert-Butyl 1,3,4,5-tetrahydro-2H-pyrido-[4,3-b]indole-2-carboxylate
- CAS Number:627869-56-1
- Molecular formula:C16H20N2O2
- Molecular Weight:272.34
Yield:627869-56-1 99%
Reaction Conditions:
with N,N'-Dimethylurea;tartaric acid at 100; for 6 h;
Steps:
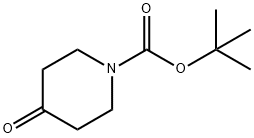
5.1 Step 1) 1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carboxylic acid tert-butyl ester
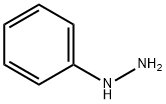
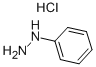
Mixing 1,3-dimethylurea (8.5 g, 95 mmol) with tartaric acid (3.6 g, 24 mmol), heat to 110 ° C until the solids melt clear, phenylhydrazine (1.1 g, 10 mmol) and N-tert-butoxycarbonyl-4-piperidone (2.0 g, 10 mmol) were added. Continue the reaction for 6 hours. The reaction was quenched by the addition of water (50 mL). Extracted with ethyl acetate (80 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated by suction. The residue was subjected to silica gel column chromatography [petroleum ether / ethyl acetate (v / v) = 5 / 1] purification, the title compound was obtained (0.24 g, yield 99%). It is a white solid.
References:
CN109928971,2019,A Location in patent:Paragraph 0272; 0274; 0275; 0276
![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole](/CAS/GIF/6208-60-2.gif)
6208-60-2
119 suppliers
$27.00/100mg
24424-99-5
840 suppliers
$13.50/25G
![tert-Butyl 1,3,4,5-tetrahydro-2H-pyrido-[4,3-b]indole-2-carboxylate](/CAS/GIF/627869-56-1.gif)
627869-56-1
13 suppliers
inquiry