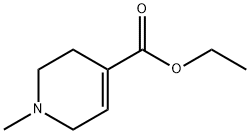
ETHYL 1-METHYL-1,2,3,6-TETRAHYDRO-4-PYRI DINECARBOXYLATE, TECH., 85 synthesis
- Product Name:ETHYL 1-METHYL-1,2,3,6-TETRAHYDRO-4-PYRI DINECARBOXYLATE, TECH., 85
- CAS Number:40175-06-2
- Molecular formula:C9H15NO2
- Molecular Weight:169.22
Yield:40175-06-2 51%
Reaction Conditions:
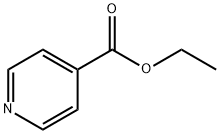
Stage #1: isonicotinic acid ethylester;methyl iodide in ethyl acetate at 80; for 3 h;
Stage #2: with sodium tetrahydroborate in ethanol at -40 - 20; for 2 h;
Steps:
1.1 (Step 1)
To a solution of ethyl isonicotinate (151 g) in ethyl acetate (1000 mL) was added methyl iodide (126 mL), and the mixture was stirred at 80° C. for 3 hr. The reaction mixture was cooled, and the orange precipitate was collected by filtration. The precipitate was suspended in ethanol (1000 mL), sodium borohydride (37.8 g) was added with cooling at -40° C., and the mixture was stirred at room temperature for 2 hr. The reaction mixture was poured into water, and ethanol was evaporated under reduced pressure. The resultant product was extracted with ethyl acetate-tetrahydrofuran, the organic layer was dried, and the solvent was evaporated under reduced pressure. The residue was distilled under reduced pressure to give ethyl 1-methyl-1,2,3,6-tetrahydropyridine-4-carboxylate (85.8 g, 51%) as a pale yellow oil.Boiling point: 64-77° C. (1 mmHg)1H-NMR (CDCl3) δ 1.29 (3H, t, J=7.2 Hz), 2.37 (3H, s), 2.40-2.50 (2H, m), 2.54 (2H, t, J=3.3 Hz), 3.09 (2H, q, J=3.0 Hz), 4.20 (2H, q, J=7.2 Hz), 6.86-6.91 (1H, m)
References:
US2009/156572,2009,A1 Location in patent:Page/Page column 37
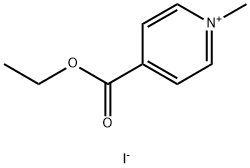
10129-59-6
8 suppliers
inquiry

40175-06-2
11 suppliers
inquiry