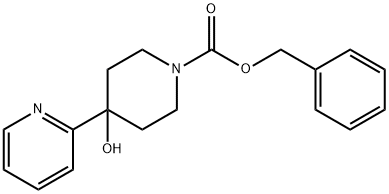
Benzyl 4-hydroxy-4-(pyridin-2-yl)piperidine-1-carboxylate synthesis
- Product Name:Benzyl 4-hydroxy-4-(pyridin-2-yl)piperidine-1-carboxylate
- CAS Number:161610-16-8
- Molecular formula:C18H20N2O3
- Molecular Weight:312.36
Yield:161610-16-8 27%
Reaction Conditions:
with n-butyllithium;NH4Cl in tetrahydrofuran;
Steps:
36.A benzyl 4-hydroxy-4-(2-pyridinyl)-1-piperidinecarboxylate
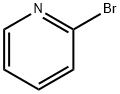
EXAMPLE 36A benzyl 4-hydroxy-4-(2-pyridinyl)-1-piperidinecarboxylate 2-Bromopyridine (0.470 mL, 5 mmol) in THF (20 mL) was treated with n-BuLi 1.6 M in hexanes (5.2 ml, 5.2 mmol) dropwise at -60° C. After stirring at -60° C. for 30 minutes, the reaction mixture and treated with benzyl 4-oxo-1-piperidinecarboxylate (1.14 g, 4.9 mmol) in THF (10 mL) slowly. After stirring an additional 15 minutes at -60° C., the reaction mixture was quenched with a saturated aqueous solution of NH4Cl, allowed to warm to room temperature and was extracted into dichloromethane. The organics were combined, dried on MgSO4, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (elution with hexanes:ethyl acetate, 1:1) to provide the title compound (400 mg, 27% yield). 1H NMR (300 MHz, DMSO-d6) δ1.54 (m, 2H), 2.05 (m, 2H), 3.25 (m, 2H), 3.95 (m, 2H), 5.11 (s, 2H), 5.35 (s, 1H), 7.25 (m, 1H), 7.35 (m, 5H), 7.68 (m, 1H), 7.79 (m, 1H), 8.5 (m, 1H); MS (DCI/NH3) m/e 313 (M+H)+.
References:
US2004/29887,2004,A1
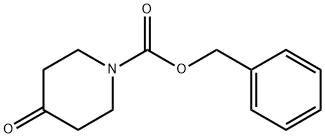
19099-93-5
318 suppliers
$5.00/1g

161610-16-8
6 suppliers
inquiry