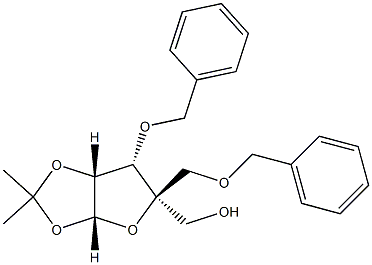
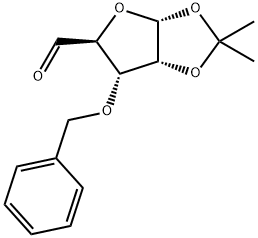
1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose synthesis
- Product Name:1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose
- CAS Number:153186-10-8
- Molecular formula:C23H28O6
- Molecular Weight:400.46
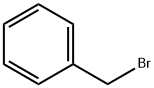
100-39-0
437 suppliers
$10.00/10g
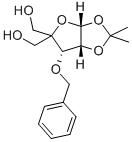
63593-03-3
152 suppliers
$38.00/250mg
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry
Yield:153186-10-8 84.2%
Reaction Conditions:
Stage #1: 1,2-O-isopropylidene-3-O-benzyl-4-hydroxymethyl-α-D-ribofuranosewith sodium hydride in N,N-dimethyl-formamide at -5; for 1 h;
Stage #2: benzyl bromide in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
1 Example 1: Synthesis of compound represented by Formula III
50.0 g (161 mmol) of the compound represented by Formula II was dissolved in 500 mL of N,N-dimethylformamide, cooled to -5°C, and 7.41 g (185 mmol) of sodium hydride (purity 60%) was added in batches. , after stirring for 1 h, add 22 mL (185 mmol) of benzyl bromide dropwise, and heat up to 20 °C and stir the reaction for 16 h. After the reaction of the raw materials is detected, add water to quench the reaction, and use dichloromethane for extraction, collect the organic phase and use cyclohexane/ Ethyl acetate was recrystallized to obtain 54.3 g of the compound represented by formula III, which was a white solid, and the yield in this step was 84.2%.
References:
CN114106063,2022,A Location in patent:Paragraph 0050-0052

63593-03-3
152 suppliers
$38.00/250mg
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry
22393-62-0
2 suppliers
inquiry
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry
63593-02-2
4 suppliers
inquiry
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry

100-39-0
437 suppliers
$10.00/10g
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry