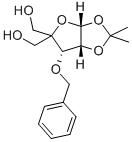
3-O-Benzyl-4-(hydroxymethyl-1,2-O-isopropylidene)-alpha-D-erythropentofuranose synthesis
- Product Name:3-O-Benzyl-4-(hydroxymethyl-1,2-O-isopropylidene)-alpha-D-erythropentofuranose
- CAS Number:63593-03-3
- Molecular formula:C16H22O6
- Molecular Weight:310.34
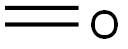
50-00-0
884 suppliers
$10.00/25g
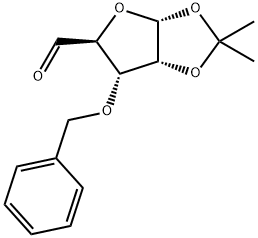
63593-02-2
5 suppliers
inquiry

63593-03-3
152 suppliers
$38.00/250mg
Yield:63593-03-3 75%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane at 28 - 30; for 16.83 h;
Steps:
3.3.5 3.5.3 Process description step 2
Dioxane (2.0 L) is added to the residue and the resulting turbid solution is filtered on a GF-P3, and the filter cake is washed with dioxane (0.3 L). The yellow clear filtrate is transferred into a reactor together with 37 % formaldehyde (700 mL). 2 N Sodium hydroxide (2.3 L) is added over a period of 50 min. The temperature is kept below 30°C by occasional cooling of the mixture. The mixture is stirred at 28°C until TLC (eluent: 1/1 EtOAc/ heptane, detected by H2SO4 + heat) shows full conversion to P-D-S-6 (typical 16 h). 3.5.4 Purification step 2 Sodium chloride (0.3 kg) is added and the mixture is stirred until the NaCl has dissolved. The phases are separated, and the aqueous phase is extracted with DCM (2 x 1.5 L). The combined organic phases are washed with 20 % sodium chloride (1.5 L). Additional sodium chloride (150 g) is added to improve phase separation. The combined organic phases are concentrated using a rotary evaporator, finally at 60°C and 30 mbar. Warm MTBE (3 L, app 50°C) is added to the residue and the hot solution (45-50°C) is transferred to a glass reactor. Heptane (1.5 L) is added and the mixture allowed to cool down to room temperature. The first crop of crystals are isolated on a GF-P3, and the filter cake is washed with MTBE/ heptane (1 :3) (1.5 L, 20°C). The filtrate is evaporated, and MTBE (500 mL) and heptane (200 mL) is added to the residue. The hot solution (45-50°C) is transferred to a flask and allowed to reach RT. The secound crop of crystals are isolated on a GF-P3. The material is left to dry in a vacuum oven over night to give 750 g (yield: 75 %) white solid. 3.5.5 Analytical method TLC (eluent: EtO Ac/heptane 1 :1): P-D-S-5: Rf= 0.20 Aldehyde intermediate (smear): Rf= 0.40 P-D-S-6: Rf= 0.20
References:
WO2019/224172,2019,A1 Location in patent:Page/Page column 39; 50-52
![(3aR,6aR)-5-[(4R)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3]dioxol-6-ol:methane](/CAS/20180529/GIF/620630-82-2.gif)
620630-82-2
3 suppliers
inquiry

63593-03-3
152 suppliers
$38.00/250mg
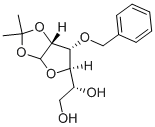
57099-04-4
20 suppliers
inquiry

63593-03-3
152 suppliers
$38.00/250mg
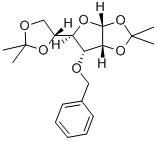
22331-21-1
85 suppliers
$70.00/10g

63593-03-3
152 suppliers
$38.00/250mg
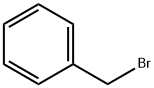
100-39-0
436 suppliers
$10.00/10g

63593-03-3
152 suppliers
$38.00/250mg