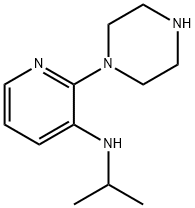
3-PYRIDYLAMINE, N-(1-METHYLETHYL)-2-(1-PIPERAZINYL)-,DIHYDROCHLORIDE MONOHYDRATE synthesis
- Product Name:3-PYRIDYLAMINE, N-(1-METHYLETHYL)-2-(1-PIPERAZINYL)-,DIHYDROCHLORIDE MONOHYDRATE
- CAS Number:147539-21-7
- Molecular formula:C12H20N4
- Molecular Weight:220.31
136818-14-9
21 suppliers
inquiry

147539-21-7
19 suppliers
inquiry
Yield:147539-21-7 100%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20; for 24.75 h;
Steps:
1 Synthesis of mPEGn-O-Delavirdine Conjugates
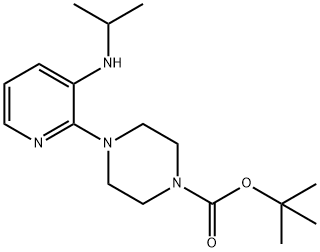
Trifluoroacetic acid (2.0 mL) was added to a stirred solution of 2-(Boc-piperazinyl)-3-(i -propylamino)pyridine 4 (1.426 g, 4.45 mmol) in dichloromethane (10 mL) at 0° C.
The resulting mixture was stirred at 0° C. for 30 min, at room temperature for 1 h 15 min.
Additional quantities of TFA (2.0 mL) were added.
The mixture was stirred at room temperature for another 23 h. Sat.
K2CO3 solution was slowly added to quench the reaction (gas ↑).
Small amount of water was added to help the layers separation.
The organic phase was separated and the aqueous phase was extracted with dichloromethane (2*25 mL).
The combined organic solution was washed with brine, dried over anhydrous Na2SO4, and concentrated to afford the product in quantitative yield. 1H-NMR (CDCl3): 7.681 (dd, J=5.0 Hz and 1.5 Hz, 1 H, Ar-H), 6.890 (dd, J=8.0 Hz and 5.0 Hz, 1 H, Ar-H), 6.800 (dd, J=8.0 Hz and 1.5 Hz, 1 H, Ar-H), 4.170 (d, J=7.5 Hz, 1 H, NH), 3.567-3.485 (m, 1 H, CH), 3.046 (s, 8 H, 4 CH2), 1.983 (br, 1 H, NH), 1.234 (d, J=6.0 Hz, 6 H, 2 CH3).
References:
US9226970,2016,B2 Location in patent:Page/Page column 29
153473-24-6
78 suppliers
$63.61/250mgs:

147539-21-7
19 suppliers
inquiry
5470-18-8
443 suppliers
$6.00/5g

147539-21-7
19 suppliers
inquiry
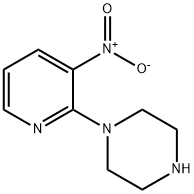
87394-48-7
68 suppliers
$14.00/100mg

147539-21-7
19 suppliers
inquiry
![3-Amino-2-[4-butoxycarbonyl(piperazino)]pyridine](/CAS/GIF/111669-25-1.gif)
111669-25-1
70 suppliers
$22.00/100mg

147539-21-7
19 suppliers
inquiry