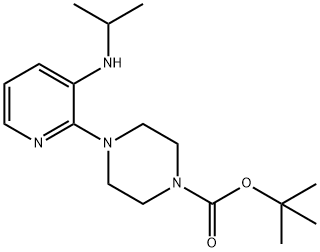
1-((1,1-DIMETHYLETHOXY)CARBONYL)-4-(3-((1-METHYLETHYL)AMINO)-2-PYRIDYL)PIPERAZINE synthesis
- Product Name:1-((1,1-DIMETHYLETHOXY)CARBONYL)-4-(3-((1-METHYLETHYL)AMINO)-2-PYRIDYL)PIPERAZINE
- CAS Number:136818-14-9
- Molecular formula:C17H28N4O2
- Molecular Weight:320.43
![3-Amino-2-[4-butoxycarbonyl(piperazino)]pyridine](/CAS/GIF/111669-25-1.gif)
111669-25-1
70 suppliers
$22.00/100mg
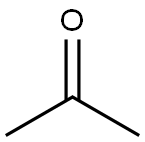
67-64-1
6 suppliers
$21.60/10ml

136818-14-9
21 suppliers
inquiry
Yield:136818-14-9 100%
Reaction Conditions:
Stage #1: 1-[1,1-dimethylethoxycarbonyl]-4-[3-amino-2-pyridinyl]piperazine;acetonewith acetic acid in 1,2-dichloro-ethane at 20; for 0.166667 h;
Stage #2: with sodium tris(acetoxy)borohydride in 1,2-dichloro-ethane at 20; for 64 h;
Steps:
1 Synthesis of mPEGn-O-Delavirdine Conjugates
3-amino-2-(Boc-piperazine)pyridine 7 (3.813 g, about 11.16 mmol, crude) was dissolved in dichloroethane (75 mL).
Acetone (1.7 mL, 23.12 mmol) was added, followed by an addition of HOAc (1.0 mL, 17.45 mmol) at room temperature.
After about 10 min, NaBH(OAc)3 (7.46 g, 33.44 mmol) was added.
An additional amount of dichloroethane (25 mL) was added.
The resulting mixture was stirred at room temperature for 64 h.
5% aqueous NaHCO3 was added to quench the reaction.
The organic phase was separated and the aqueous phase was extracted with dichloromethane (2*50 mL).
The combined organic solution was washed with brine, dried over anhydrous Na2SO4, concentrated.
The residue was purified via flash column chromatography over silica gel with 5-35% ethyl acetate in hexane to afford the product as solid in quantitative yield. 1H-NMR (CDCl3): 7.673 (dd, J=5.0 Hz and 1.5 Hz, 1 H, Ar-H), 6.904 (dd, J=8.0 Hz and 5.0 Hz, 1 H, Ar-H), 6.812 (dd, J=8.0 Hz and 1.5 Hz, 1 H, Ar-H), 4.150 (d, J=7.5 Hz, 1 H, NH), 3.569-3.517 (m, 5 H, 2CH2 and CH), 2.997 (t, J=5.0 Hz, 4 H, 2 CH2), 1.477 (s, 9 H, 3 CH3), 1.23 (d, J=6.0 Hz, 6 H, 2 CH3).
(ref.
Romero D. L. et al. J. Med. Chem. 37, 999-1014, 1994.)
Synthesis of 2-piperazinyl-3-(isopropylamino)pyridine 8:
References:
US9226970,2016,B2 Location in patent:Page/Page column 28; 29

67-64-1
6 suppliers
$21.60/10ml
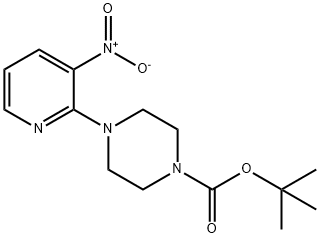
153473-24-6
78 suppliers
$63.61/250mgs:

136818-14-9
21 suppliers
inquiry

153473-24-6
78 suppliers
$63.61/250mgs:

136818-14-9
21 suppliers
inquiry
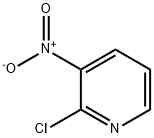
5470-18-8
445 suppliers
$6.00/5g

136818-14-9
21 suppliers
inquiry
87394-48-7
68 suppliers
$14.00/100mg

136818-14-9
21 suppliers
inquiry