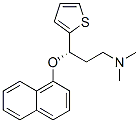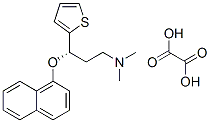
S-(+)-N,N-Dimethyl-3-(1-naphthoxy)-3-(2-thienyl)-1-propylamine oxalate synthesis
- Product Name:S-(+)-N,N-Dimethyl-3-(1-naphthoxy)-3-(2-thienyl)-1-propylamine oxalate
- CAS Number:132335-47-8
- Molecular formula:C21H23NO5S
- Molecular Weight:401.48
Yield:132335-47-8 84.2%
Reaction Conditions:
in Isopropyl acetate;water at 20; for 21 h;Product distribution / selectivity;
Steps:
10
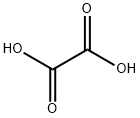
Example 10; Preparation of (S)-N,N-dimethyl-(3-(1-naphthyloxy)-3-thien-2-yl)propylamine Oxalic Acid Salt(S)-N,N-dimethyl-(3-(1-naphthyloxy)-3-thien-2 yl)propylamine (10 g, 32.1 mmoles) was dissolved in isopropyl acetate (50 mL) at ambient temperature. A solution of oxalic acid dihydrate (3.64 g, 25.7 moles, 0.8 eq) in water (30 mL) was then added. The resulting mixture was stirred for 21 hours and filtered. The filter cake was washed with isopropyl acetate (10 mL) and dried under vacuum at 40° C. to yield 10.87 g of the product as a white solid (Yield: 84.2%; HPLC (peak area at 220 nm) oxalic acid 1.78%, 4-[3-dimethylamino-1-(2-thienyl)-1-propyl]naphthol 0.10%, 1-naphthol 0.35%, (S)-N,N-dimethyl-(3-(1-naphthyloxy)-3-thien-2-yl)propylamine 97.65%; Titration: 99.5%; Karl Fischer: 0.06%; XRD as shown in FIG. 1 (Form A); IR essentially as shown in FIG. 3 (Form A); TGA DSC as shown in FIG. 2, mp onset 152.6° C.).
References:
US2009/93645,2009,A1 Location in patent:Page/Page column 6
321-38-0
604 suppliers
$6.00/10g
144-62-7
787 suppliers
$5.00/5g
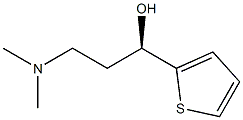
132335-49-0
35 suppliers
inquiry

132335-47-8
190 suppliers
inquiry

321-38-0
604 suppliers
$6.00/10g

144-62-7
787 suppliers
$5.00/5g
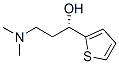
132335-44-5
343 suppliers
$6.00/1g

132335-47-8
190 suppliers
inquiry

321-38-0
604 suppliers
$6.00/10g

132335-47-8
190 suppliers
inquiry

144-62-7
787 suppliers
$5.00/5g
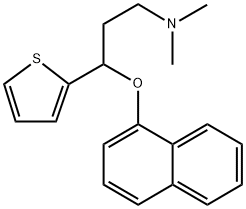
116817-11-9
90 suppliers
inquiry
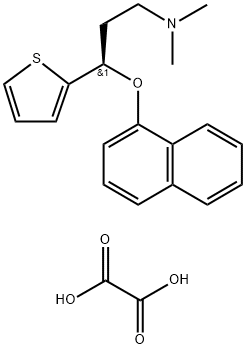
932013-45-1
11 suppliers
inquiry

132335-47-8
190 suppliers
inquiry