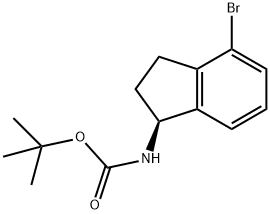
Carbamic acid, N-[(1S)-4-bromo-2,3-dihydro-1H-inden-1-yl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[(1S)-4-bromo-2,3-dihydro-1H-inden-1-yl]-, 1,1-dimethylethyl ester
- CAS Number:1307231-21-5
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2
24424-99-5
842 suppliers
$13.50/25G
1307873-37-5
36 suppliers
$129.00/100mg
![Carbamic acid, N-[(1S)-4-bromo-2,3-dihydro-1H-inden-1-yl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1307231-21-5.gif)
1307231-21-5
7 suppliers
inquiry
Yield:1307231-21-5 14 g
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
(S)-tert-butyl 4-bromo-2,3-dihydro-1H-inden-1-ylcarbamate (IND INT-15)
(S)-tert-butyl 4-bromo-2,3-dihydro-1H-inden-1-ylcarbamate (IND INT-15)
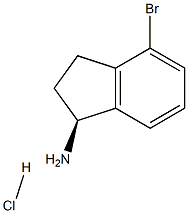
To crude (S)-4-bromo-2,3-dihydro-1H-inden-1-amine hydrochloride IND INT-13 (16.6 g, 66 mmol) in DCM (140 mL) at 0° C. was added triethylamine (14.8 g, 146 mmol) and di-tert-butyl dicarbonate (16.0 g, 73 mmol).
The reaction was stirred at room temperature overnight.
The reaction was diluted with DCM (50 mL) and washed with water and brine.
The organic layers were dried over MgSO4 and the product purified by crystallization from 10% EA/hexanes to afford 14 g of (70%) (S)-tert-butyl 4-bromo-2,3-dihydro-1H-inden-1-ylcarbamate IND INT-15 as an off-white solid. LCMS-ESI (m/z) calculated for C14H18BrNO2: 312.2. found 197.0 [M-NH2Boc]+, tR=3.94 min. 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J=7.9 Hz, 1H), 7.25 (d, J=7.8 Hz, 1H), 7.08 (t, J=7.7 Hz, 1H), 5.25 (dd, J=15.9, 7.9 Hz, 1H), 4.78 (d, J=7.6 Hz, 1H), 2.99 (ddd, J=16.5, 9.0, 3.4 Hz, 1H), 2.81 (dt, J=16.5, 8.2 Hz, 1H), 2.70-2.36 (m, 1H), 1.94-1.71 (m, 1H), 1.47 (d, J=5.2 Hz, 9H).
13C NMR (101 MHz, CDCl3) δ 155.99, 146.13, 143.83, 131.35, 129.02, 123.41, 120.64, 80.10, 57.21, 33.71, 31.82, 28.86; Chiral HPLC: (S)-tert-butyl 4-bromo-2,3-dihydro-1H-inden-1-ylcarbamate was eluted using 2% IPA in hexanes: >99.9% ee, tR=11.08 min.
(R)-tert-butyl 4-bromo-2,3-dihydro-1H-inden-1-ylcarbamate IND INT-16 was prepared in an analogous fashion from (R)-4-bromo-2,3-dihydro-1H-inden-1-amine hydrochloride IND INT-14: >99.9% ee tR for (R)-enantiomer=9.98 min.
References:
US2015/299179,2015,A1 Location in patent:Paragraph 0299; 0300