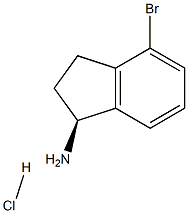
(S)-4-Bromo-2,3-dihydro-1H-inden-1-amine hydrochloride synthesis
- Product Name:(S)-4-Bromo-2,3-dihydro-1H-inden-1-amine hydrochloride
- CAS Number:1307873-37-5
- Molecular formula:C9H11BrClN
- Molecular Weight:248.5473
1307231-20-4
4 suppliers
inquiry

1307873-37-5
36 suppliers
$129.00/100mg
Yield:-
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;methanol at 20; for 3 h;
Steps:
(S)-4-bromo-2,3-dihydro-1H-inden-1-amine hydrochloride (IND INT-13)
(S)-4-bromo-2,3-dihydro-1H-inden-1-amine hydrochloride (IND INT-13)
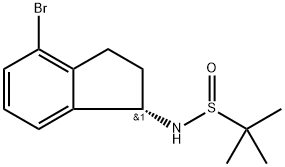
To a stirred suspension of crude (S)-N-((S)-4-bromo-2,3-dihydro-1H-inden-1-yl)-2-methylpropane-2-sulfinamide IND INT-11 (46 g, 145 mol) in MeOH (100 mL) was added 4N HCl in dioxane (109 mL) and the yellow suspension was stirred at room temperature for 3 h.
The crude reaction was diluted with MeOH (100 mL) and filtered.
The filtrate was concentrated and the solid obtained was dispersed into acetonitrile (600 mL) and refluxed for 90 min.
The suspension was cooled to 0° C. and the solid filtered to produce 25 g of (69%) (S)-4-bromo-2,3-dihydro-1H-inden-1-amine hydrochloride IND INT-13 which was used in the next step without purification. LCMS-ESI (m/z) calculated for C9H10BrN: 211.09. found 197.0 [M-NH2]+, tR=1.76 min. 1H NMR (400 MHz, DMSO) δ 8.76 (s, 2H), 7.71 (d, J=7.5 Hz, 1H), 7.57 (d, J=7.9 Hz, 1H), 7.26 (t, J=7.7 Hz, 1H), 4.80 (s, 1H), 3.06 (ddd, J=16.9, 8.9, 5.2 Hz, 1H), 2.93-2.76 (m, 1H), 2.57-2.39 (m, 1H), 2.11-1.92 (m, 1H); 13C NMR (101 MHz, DMSO) δ 144.12, 141.60, 131.71, 129.02, 124.54, 119.29, 55.30, 31.52, 29.10.
References:
US2015/299179,2015,A1 Location in patent:Paragraph 0296; 0297