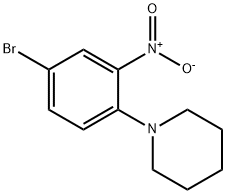
1-(4-bromo-2-nitrophenyl)piperidine synthesis
- Product Name:1-(4-bromo-2-nitrophenyl)piperidine
- CAS Number:5465-66-7
- Molecular formula:C11H13BrN2O2
- Molecular Weight:285.14
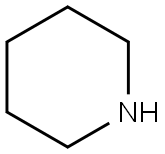
110-89-4
0 suppliers
$15.96/100ML
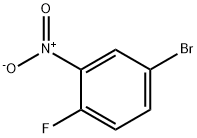
364-73-8
273 suppliers
$8.00/10g

5465-66-7
13 suppliers
$28.60/250mg
Yield:5465-66-7 99%
Reaction Conditions:
with potassium carbonate in (methylsulfinyl)methane at 100;
Steps:
4.1.6. General procedure for the preparation of compounds 11a-g and 12f-g
General procedure: Alkylamine (30mmol) and K2CO3 (4.1g, 30mmol) or CsCO3 (9.8g, 30mmol) was added to compound 10 (10mmol) in DMF or DMSO (20mL). The mixture was heated at 25-120°C for 2-12h. The reaction was diluted with H2O (200mL) and then extracted with EtOAc (200mL×2). The combined organic phases were washed with H2O (200mL×2) and brine (200mL), dried over MgSO4 and concentrated in a vacuum. The residue was purified by flash column chromatography (0-10% EtOAc in petroleum ether).
References:
Li, Jing;Wu, Fangping;Liu, Xingguo;Zou, Yake;Chen, Huixiong;Li, Zheng;Zhang, Lei [European Journal of Medicinal Chemistry,2017,vol. 140,p. 20 - 30]

110-89-4
0 suppliers
$15.96/100ML
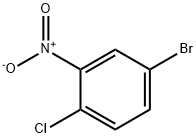
16588-24-2
153 suppliers
$6.00/1g

5465-66-7
13 suppliers
$28.60/250mg

110-89-4
0 suppliers
$15.96/100ML
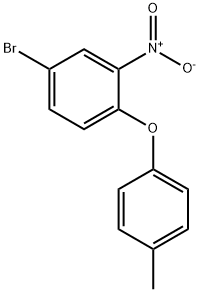
55321-64-7
4 suppliers
inquiry
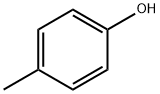
106-44-5
497 suppliers
$14.00/5g

5465-66-7
13 suppliers
$28.60/250mg

110-89-4
0 suppliers
$15.96/100ML
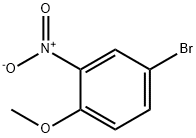
33696-00-3
202 suppliers
$10.00/1g
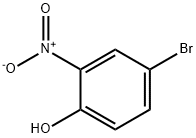
7693-52-9
236 suppliers
$5.00/1g

5465-66-7
13 suppliers
$28.60/250mg

110-89-4
0 suppliers
$15.96/100ML

55321-64-7
4 suppliers
inquiry
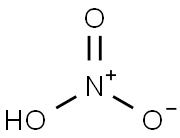
7697-37-2
0 suppliers
$13.00/25g
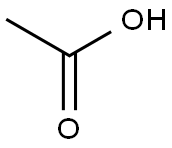
64-19-7
1585 suppliers
$10.00/25ML
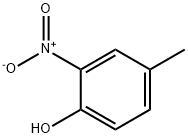
119-33-5
13 suppliers
$23.00/25g

5465-66-7
13 suppliers
$28.60/250mg