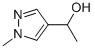
1-(1-Methyl-1H-pyrazol-4-yl)ethanol synthesis
- Product Name:1-(1-Methyl-1H-pyrazol-4-yl)ethanol
- CAS Number:40534-33-6
- Molecular formula:C6H10N2O
- Molecular Weight:126.16
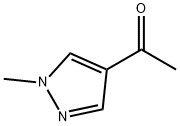
37687-18-6
97 suppliers
$25.00/1g

40534-33-6
54 suppliers
$65.00/100mg
Yield:40534-33-6 159 mg
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 20;
Steps:
Synthesis of N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-methyl-1H-pyrazol-4-yl)ethyl)-1H-indole-3-carboxamide (12)
1-(1-Methyl-1H-pyrazol-4-yl)ethan-1-ol. To a resealable vial was added 4-iodo-1-methyl-1H-pyrazole (772 mg, 3.71 mmol), THF (6 mL), and the solution was cooled to 0 °C. To this solution was added isopropylmagnesium chloride lithium chloride complex solution (3.42 mL, 4.45 mmol) and the reaction stirred for 30 min (complete disappearance of iodo-pyrazole as detected via LCMS) before cooling to -78 °C and addition of N-methoxy-N-methylacetamide (1.18 mL, 11.13 mmol). The solution was stirred at -78 °C for 1 h before removing the cold bath and warming to ambient temperature. The solution was diluted with EtOAc and quenched via the addition of 1N HCl. The layers were separated and the aqueous was extracted with EtOAc. The combined organics layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The crude residue was taken up in MeOH and cooled to 0 °C before addition of NaBH4 (281 mg, 7.42 mmol). The reaction was stirred while warming to ambient temperature and quenched when LCMS showed disappearance of starting material. This solution was then diluted with 1N HCl and EtOAc. The layers were separated and the aqueous was extracted with EtOAc (3X). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The crude material was purified via silica gel chromatography (MeOH:DCM) to afford 1-(1-methyl-1H-pyrazol-4-yl)ethanol (159 mg, 1.260 mmol, 34.0 % yield). LC-MS m/z 127 [M + H]+.
References:
Gehling, Victor S.;Vaswani, Rishi G.;Nasveschuk, Christopher G.;Duplessis, Martin;Iyer, Priyadarshini;Balasubramanian, Srividya;Zhao, Feng;Good, Andrew C.;Campbell, Robert;Lee, Christina;Dakin, Les A.;Cook, Andrew S.;Gagnon, Alexandre;Harmange, Jean-Christophe;Audia, James E.;Cummings, Richard T.;Normant, Emmanuel;Trojer, Patrick;Albrecht, Brian K. [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 17,p. 3644 - 3649] Location in patent:supporting information
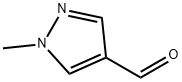
25016-11-9
241 suppliers
$5.00/1g
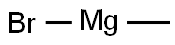
75-16-1
270 suppliers
$12.00/10ml

40534-33-6
54 suppliers
$65.00/100mg