|
|
| | Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid Basic information | | Physical Form |
| Product Name: | Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid | | Synonyms: | Benzenebutanoicacid,b-[[(1,1-diMethylethoxy)carbonyl]aMino]-2,4,5-trifluoro-,(bR)-;(R)-Sitagliptin N-Boc-Acid IMpurity;Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid (3R)-N-(tert-Butoxycarbonyl)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid;Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid, >=99%;(3R)-N-Boc-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid;(3R)-N-(tert-Butoxycarbonyl)-3-aMino-4-(2,4,5-trifluorophenyl)-butanoic acid- Boc-(R)-3-AMino-4-(2,4,5-trifluorophenyl)
butanoic acid (BOC acid);[[(1,1-diMethylethoxy)carbonyl]aMino]-2,4,5-trifluoro-(R)-benzenebuta;BOC-(R)-3-AMINO-4-(2,4,5-TRIFLUORO-PHENYL)-BUTYRIC ACID | | CAS: | 486460-00-8 | | MF: | C15H18F3NO4 | | MW: | 333.31 | | EINECS: | 691-120-8 | | Product Categories: | B-Amino | | Mol File: | 486460-00-8.mol | 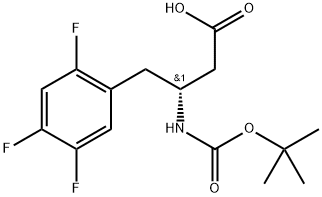 |
| | Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid Chemical Properties |
| Melting point | 136-138℃ | | Boiling point | 443.1±45.0 °C(Predicted) | | density | 1.292±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | form | Solid | | pka | 4.30±0.10(Predicted) | | color | White to Off-White |
| | Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid Usage And Synthesis |
| Physical Form | White to Yellow Solid | | Description | Boc-(R) -3-amino-4 -(2,4, 5-trifluorophenyl) butyric acid is an important intermediate for the preparation of sitagliptin. Sitagliptinphosphate is the first dipeptidase -IV(dpp-4) inhibitor approved by FDA in 2006. It is used for the treatment of type II diabetes mellitus. It has obvious hypoglycemic effect when used alone or in combination with metformin and pioglitazone, and it is safe to take, well tolerated, with few adverse reactions. | | Uses | (R)-3-((tert-Butoxycarbonyl)amino)-4-(2,4,5-trifluorophenyl)butanoic Acid has been used as a reactant for the preparation of dipeptidyl peptidase-4 (DPP4) inhibitors for the treatment of type 2 diabetes, such as sitagliptin. | | Synthesis | (R)-β-tert-butoxycarbonylamino-2,4,5-trifluorophenylbutyric acid ethyl ester could used as a starting material and followed by adding Methanol and LiOHH2O as reaction reagents. Adding dichloromethane to the concentrate and adjust the pH to 2 with 2N HCl. Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid could be obtained through further purification. |
| | Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid Preparation Products And Raw materials |
|