|
|
| | AMMONIUM IRON (III) HEXACYANOFERRATE (II) Basic information |
| Product Name: | AMMONIUM IRON (III) HEXACYANOFERRATE (II) | | Synonyms: | afcf;ammonium-ferric-cyano-ferrate(ii);ammonium-ferric-ferrocyanide;ammoniumiron(3+)(1:1:1),(oc-6-11)-ferrate(4-hexakis(cyano-c)-;ammoniumiron(3++)(1:1:1),(oc-6-11)-ferrate(4-hexakis(cyano-c)-;ammoniumiron(iii)hexacyanoferrate;ammoniumironhexacyanoferrate;ferricammoniumferrocyanide | | CAS: | 25869-00-5 | | MF: | C6H4Fe2N7 | | MW: | 285.84 | | EINECS: | 247-304-1 | | Product Categories: | Inorganics | | Mol File: | 25869-00-5.mol | 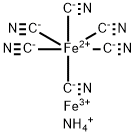 |
| | AMMONIUM IRON (III) HEXACYANOFERRATE (II) Chemical Properties |
| RTECS | LJ8140000 | | Water Solubility | Soluble in water and mineral acids. Insoluble in alkali. | | Merck | 14,7910 | | Exposure limits | ACGIH: TWA 1 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 1 mg/m3 | | LogP | -0.250 (est) | | EPA Substance Registry System | Ferric ammonium ferrocyanide (25869-00-5) |
| Risk Statements | 20/21/22 | | Safety Statements | 22-36/37/39 | | TSCA | Yes | | Toxicity | LD50 orally in mice: >5 g/kg (Pearce) |
| Provider | Language |
|
ALFA
| English |
| | AMMONIUM IRON (III) HEXACYANOFERRATE (II) Usage And Synthesis |
| Uses | It is employed as a intermediate for pharmaceuticals. Also used in decorative cosmetics. | | General Description | Solid. | | Air & Water Reactions | Irradiation of aqueous solutions of the compound will liberate hydrocyanic acid. | | Reactivity Profile | AMMONIUM IRON (III) HEXACYANOFERRATE (II) is an inorganic cyanide. Inorganic cyanides react slowly with water to evolve gaseous hydrogen cyanide (HCN). Acids cause the rapid evolution of HCN; carbon dioxide from the air is sufficiently acidic to liberate HCN from solutions of cyanides. Inorganic cyanides are incompatible with isocyanates, nitrides, and peroxides. Cyanides have been known to initiate polymerization reactions of epoxides. Cyanides form compounds with metal salts; heat and hydrogen production may accompany these reactions. Some cyanides can detonate when exposed to shock, heat, or friction. In the presence of strong acids or acid fumes may release highly toxic fumes of cyanides. | | Fire Hazard | Flash point data for AMMONIUM IRON (III) HEXACYANOFERRATE (II) are not available. AMMONIUM IRON (III) HEXACYANOFERRATE (II) is probably combustible. |
| | AMMONIUM IRON (III) HEXACYANOFERRATE (II) Preparation Products And Raw materials |
|