|
|
| | Cefuroxime Sodium EP Impurity C Basic information |
| Product Name: | Cefuroxime Sodium EP Impurity C | | Synonyms: | Cefuroxime Sodium EP Impurity C;Cefuroxime Sodium Impurity C (EP);Cefuroxime Impurity 3(Cefuroxime Sodium EP Impurity C);(6R,7R)-7-((Z)-2-(furan-2-yl)-2-(methoxyimino)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;[6R-[6α,7β(Z)]]- 7-[[2-furanyl(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid;Cefuroxime EP Impurity C;Cefuroxime Impurity 3/Cefuroxime EP Impurity C/(6R,7R)-7-[[(Z)-(furan-2-yl)(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-2-(2-furanyl)-2-(methoxyimino)acetyl]amino]-3-methyl-8-oxo-, (6R,7R)- | | CAS: | 69822-88-4 | | MF: | C15H15N3O6S | | MW: | 365.36 | | EINECS: | | | Product Categories: | | | Mol File: | 69822-88-4.mol | 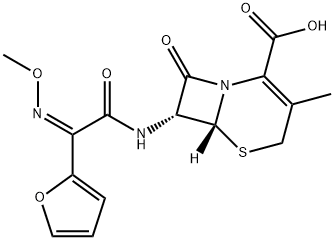 |
| | Cefuroxime Sodium EP Impurity C Chemical Properties |
| density | 1.63±0.1 g/cm3(Predicted) | | pka | 3.11±0.50(Predicted) |
| | Cefuroxime Sodium EP Impurity C Usage And Synthesis |
| | Cefuroxime Sodium EP Impurity C Preparation Products And Raw materials |
|