| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:4'-METHOXYVALEROPHENONE
CAS:1671-76-7
|
| Company Name: |
Yangzhou Siyu Chemical Co.,Ltd.
|
| Tel: |
0514-87325867 |
| Email: |
sales@siyuchem.com |
| Products Intro: |
Product Name:4'-Methoxy-valerophenone
CAS:1671-76-7
Purity:98% Package:1g,10g,50g,100g,500g,1kg
|
|
| | 4'-METHOXYVALEROPHENONE Basic information |
| Product Name: | 4'-METHOXYVALEROPHENONE | | Synonyms: | P-METHOXYVALEROPHENONE;1-(4-METHOXY-PHENYL)-PENTAN-1-ONE;4-METHOXYVALEROPHENONE;1-(4-Methoxyphenyl)-1-pentanone;1-(4-Methoxyphenyl)pentane-1-one;4-Methoxy-1-phenylpentan-1-one;1-Pentanone, 1-(4-methoxyphenyl)-;p-Methoxyvalerophenon | | CAS: | 1671-76-7 | | MF: | C12H16O2 | | MW: | 192.25 | | EINECS: | 216-803-6 | | Product Categories: | | | Mol File: | 1671-76-7.mol | 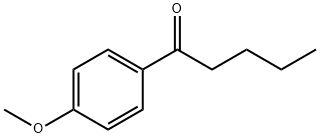 |
| | 4'-METHOXYVALEROPHENONE Chemical Properties |
| | 4'-METHOXYVALEROPHENONE Usage And Synthesis |
| Preparation | To a solution of N-methoxy-Nmethyl- 2-pyridyl urethane (364.4 mg, 2 mmol) in THF (6 mL), p-methoxyphenylmagnesium bromide (0.25 m in THF, 8 mL, 2 mmol) was added dropwise over a period of 10 min at 0 ℃ under nitrogen atmosphere. Stirring was maintained for a further 5 min and then n-butyllithium (1.60 M in hexane, 1.5 mL, 2.4 mmol) was added directly to the mixture (one-pot process). The reaction mixture was stirred for 0.5 h, while warming to room temperature, and was then quenched with 1 N HCl (5 mL). After evaporation of the THF, the concentrated mixture was poured into 1 N HCl (30 mL) and the aqueous phase was extracted with dichloromethane (3×20 mL). The combined organic extracts were dried over MgSO4, filtered, and concentrated to dryness in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/n-hexane, 1:4) to give 369.1 mg (96%) of p-methoxyvalerophenone. | | Preparation | To a solution of N-methoxy-N-methyl-2-pyridyl urethane (364.4 mg, 2 mmol) in THF (6 mL), p-methoxyphenylmagnesium bromide (0.25 m in THF, 8 mL, 2 mmol) was added dropwise over a period of 10 min at 0 °C under nitrogen atmosphere. Stirring was maintained for a further 5 min and then n-butyllithium (1.60 m in hexane, 1.5 mL, 2.4 mmol) was added directly to the mixture (one-pot process). The reaction mixture was stirred for 0.5 h, while warming to room temperature, and was then quenched with 1 n HCl (5 mL). After evaporation of the THF, the concentrated mixture was poured into 1 n HCl (30 mL) and the aqueous phase was extracted with dichloromethane (3*20 mL). The combined organic extracts were dried over MgSO4, filtered, and concentrated to dryness in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/n-hexane, 1:4) to give 369.1 mg (96%) of p-methoxyvalerophenone. | | Synthesis Reference(s) | Chemistry Letters, 15, p. 165, 1986
Tetrahedron, 50, p. 11839, 1994 DOI: 10.1016/S0040-4020(01)89299-1 |
| | 4'-METHOXYVALEROPHENONE Preparation Products And Raw materials |
|