| Structure |
Chemical Name |
CAS |
MF |
 |
Landiolol impurity S |
|
|
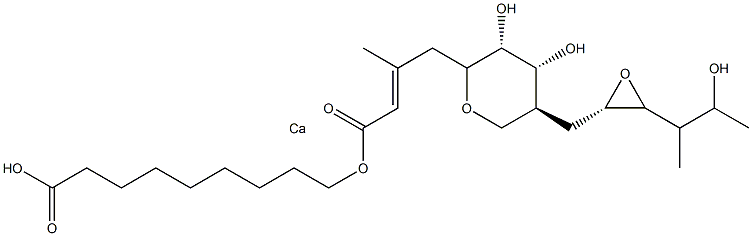 |
Mupirocin Calcium EP Impurity G |
|
C26H44CaO9 |
 |
Hyoscine Butylbromide EP Impurity F |
|
|
 |
Tocopherol EP Impurity D |
|
|
 |
Org246653-1(RRT0.35) |
|
|
![1H-Isoindole-1,3(2H)-dione, 2-[(2S)-2,3-dihydroxypropyl]-](/CAS/20200401/GIF/119835-88-0.gif) |
1H-Isoindole-1,3(2H)-dione, 2-[(2S)-2,3-dihydroxypropyl]- |
119835-88-0 |
C11H11NO4 |
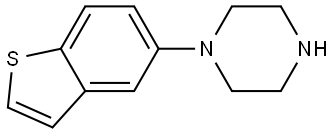 |
Brexpiprazole Impurity 76 |
433303-94-7 |
C12H14N2S |
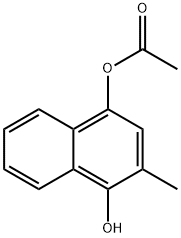 |
1,4-Naphthalenediol, 2-methyl-, 4-acetate |
110327-29-2 |
C13H12O3 |
 |
Oseltamivir Impurity 105 |
|
|
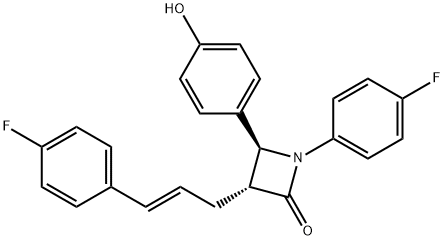 |
Ezetimibe Related Impurity 37 |
204589-68-4 |
C24H19F2NO2 |
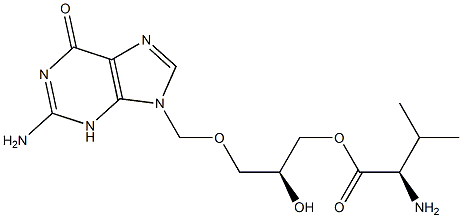 |
S, R-Isovalganciclovir Impurity |
1356932-18-7 |
C14H22N6O5 |
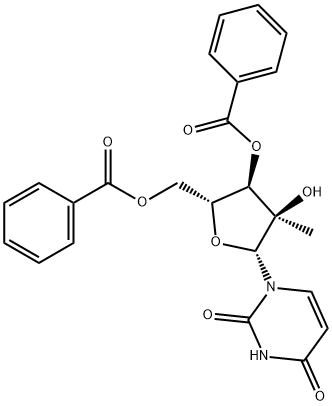 |
Sofosbuvir Impurity 8 |
944476-44-2 |
C24H22N2O8 |
![1-((2'-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzo[d]imidazole-6-carboxylic acid](/CAS/20180703/GIF/1098100-87-8.gif) |
1-((2'-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzo[d]imidazole-6-carboxylic acid |
1098100-87-8 |
C26H23N3O2 |
 |
Fimasartan Impurity |
|
|
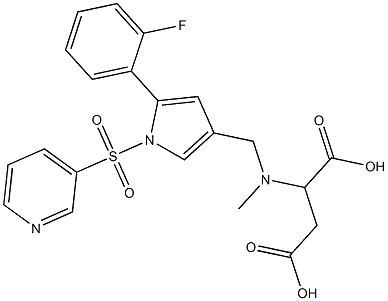 |
2-(((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)amino)succinic acid |
|
C21H20FN3O6S |
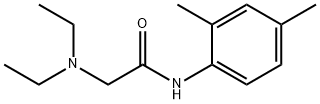 |
2-(DIETHYLAMINO)-N-(2,4-DIMETHYLPHENYL)ACETAMIDE |
17289-53-1 |
C14H22N2O |
![(3RS)-3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one](/CAS/20180703/GIF/CB72920112.gif) |
(3RS)-3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one |
|
C16H20N2O |
 |
Oxaliplatin impurity |
|
|
 |
Febuxostat Impurity 78 |
|
|
 |
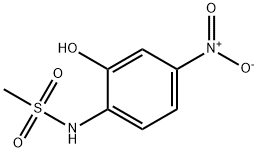
Ibrutinib Impurity 21 |
1642571-08-1 |
C27H30N6O3 |
 |
NiMesulide EP IMpurity |
38880-53-4 |
C7H8N2O5S |
 |
Moxifloxacin Impurities |
|
|
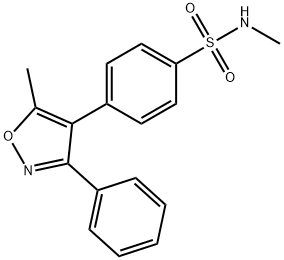 |
Parecoxib Impurity K |
198471-69-1 |
C17H16N2O3S |
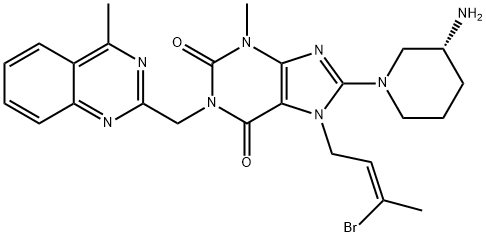 |
Linagliptin Impurity K |
1638744-06-5 |
C25H29BrN8O2 |
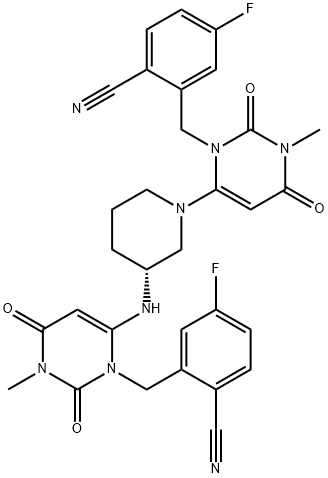 |
(R)-2-((6-(3-((3-(2-cyano-5-fluorobenzyl)-1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)amino)piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)-4-fluorobenzonitrile |
1917324-13-0 |
C31H28F2N8O4 |
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif) |
L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1) |
1194550-65-6 |
C27H27Cl3N2O5S |
 |
Etoricoxib Impurity 14 |
|
|
 |
Riociguat Impurtiy 3 |
|
|
 |
Trelagliptin Impurity 2 |
|
|
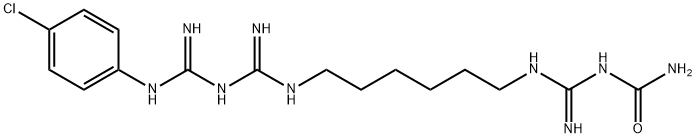 |
クロルヘキシジン二塩酸塩不純物B |
1308292-89-8 |
C16H26ClN9O |
 |
Dapoxetine Impurity D |
|
|
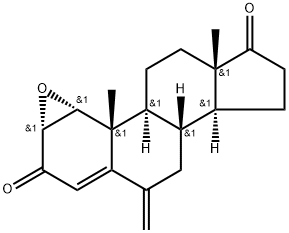 |
Exemestane Impurity 1 |
159354-61-7 |
C20H24O3 |
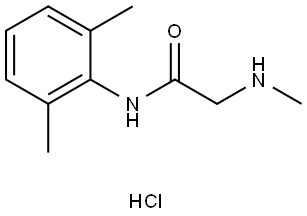 |
Lidocaine Impurity |
35891-84-0 |
C11H16N2O.ClH |
 |
Pramipexole Impurity J |
|
|
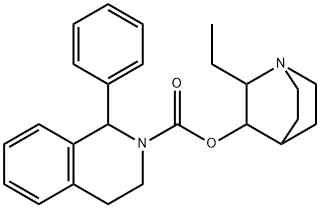 |
Solifenacin Related Compound 14 |
605696-10-4 |
C25H30N2O2 |
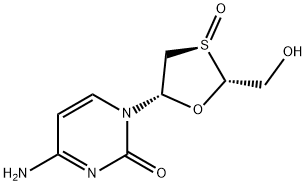 |
4-amino-1-((2R,3R,5S)-2-(hydroxymethyl)-3-oxido-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one |
160552-54-5 |
C8H11N3O4S |
![8-[(1R)-1-Hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-6-(phenylmethoxy)-2H-1,4-benzoxazin-3(4H)-one](/CAS/20180703/GIF/869478-13-7.gif) |
8-[(1R)-1-Hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-6-(phenylmethoxy)-2H-1,4-benzoxazin-3(4H)-one |
869478-13-7 |
C28H32N2O5 |
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif) |
1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate |
743460-48-2 |
C21H27N3O2 |
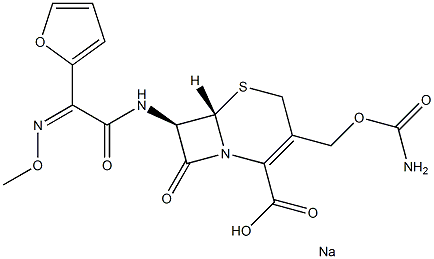 |
Cefuroxime Sodium impurity D |
|
C16H16N4NaO8S |
 |
Silodosin impurity A |
|
|
 |
cefditoren △-3 isomer |
|
|
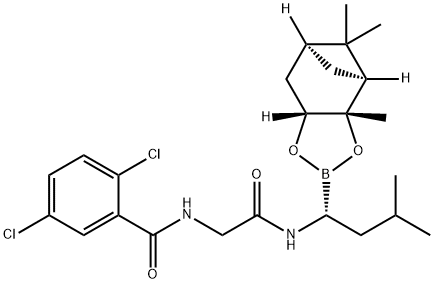 |
Ixazomib Impurity 1 |
1201903-02-7 |
C24H33BCl2N2O4 |
![(3S)-9,10-difluoro-3-methyl-7-oxo-2,3,6,7-tetrahydro-5H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid](/CAS/20180703/GIF/1026952-91-9.gif) |
(3S)-9,10-difluoro-3-methyl-7-oxo-2,3,6,7-tetrahydro-5H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid |
1026952-91-9 |
C13H11F2NO4 |
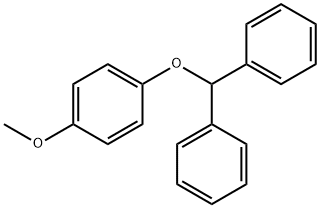 |
((4-Methoxyphenoxy)methylene)dibenzene |
85686-16-4 |
C20H18O2 |
 |
Tofacitinib Impurity L |
|
|
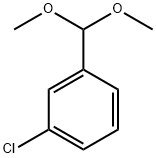 |
1-クロロ-3-(ジメトキシメチル)ベンゼン |
3395-80-0 |
C9H11ClO2 |
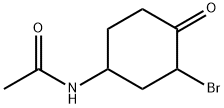 |
N-(3-ブロモ-4-オキソシクロヘキシル)アセトアミド |
687639-03-8 |
C8H12BrNO2 |
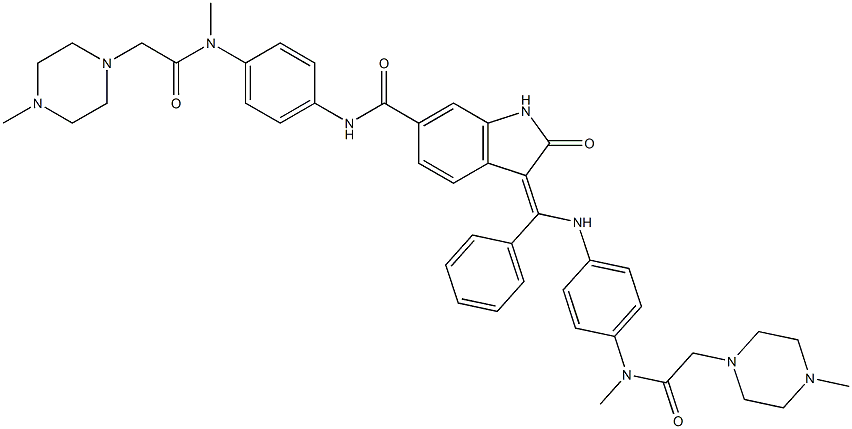 |
methyl (Z)-3-(hydroxy(phenyl)methylene)-2-oxoindoline-6-carboxylate |
|
C44H51N9O4 |
 |
Alvimopan Impurity 5 |
|
|
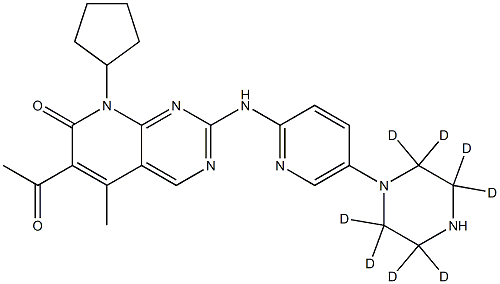 |
Palbociclib-d8 |
1628752-83-9 |
C24H21D8N7O2 |
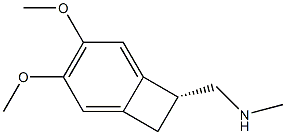 |
Ivabradine Impurity 15 |
1132667-04-9 |
C12H17NO2 |
 |
Sofosbuvir Impurity27 |
|
|
 |
Isavuconazole Impurity 12 |
|
|
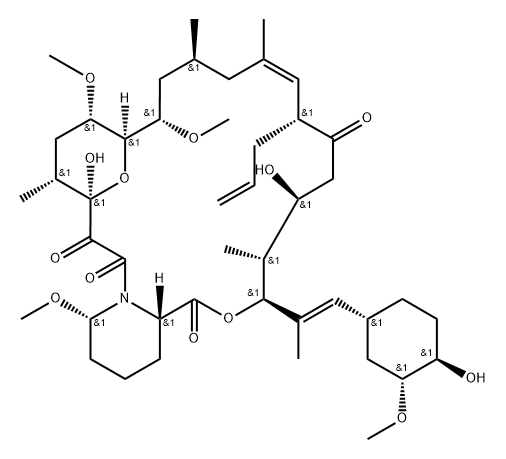 |
Tacrolimus Impurity 3 |
161861-10-5 |
C45H71NO13 |
 |
Lenvatinib Impurity 22 |
|
|
 |
Moxifloxacin Impurity 47 |
|
|
 |
Parecoxib sodium Impurity 35 |
|
|
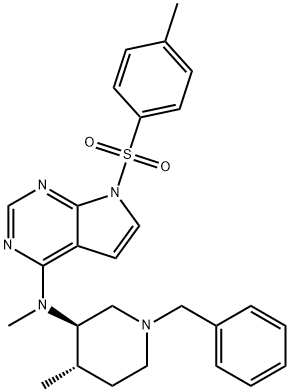 |
Tofacitinib Impurity 20 |
1092578-44-3 |
C27H31N5O2S |
 |
Tofacitinib Impurity 88 |
|
|
 |
Vildagliptin Impurity ZA9 |
|
|
 |
Nintedanib Impurity K |
|
|
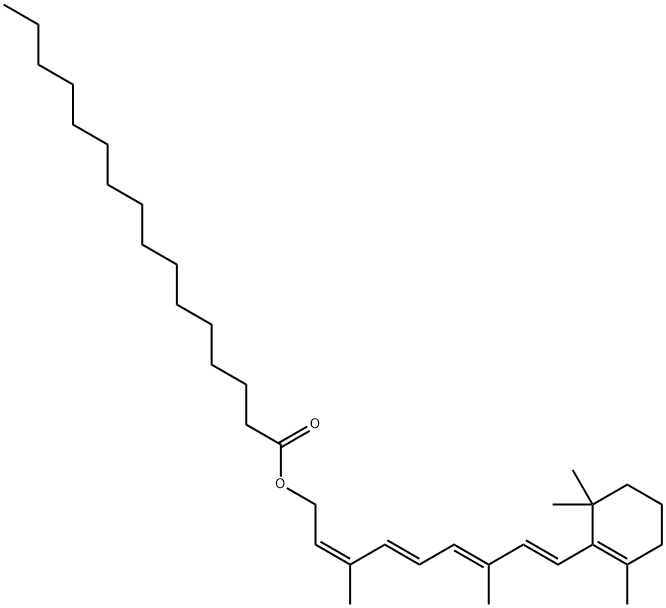 |
13-Cis Vitamin A Palmitate |
26771-20-0 |
C36H60O2 |
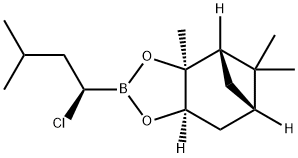 |
Bortezomib Impurity 52 |
87304-47-0 |
C15H26BClO2 |
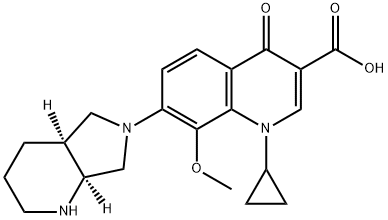 |
Moxifloxacin Impurity 20 HCl |
959347-97-8 |
C21H25N3O4 |
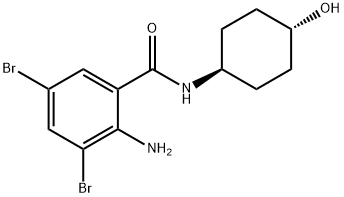 |
2-amino-3,5-dibromo-N-((1r,4r)-4-hydroxycyclohexyl)benzamide |
105735-86-2 |
C13H16Br2N2O2 |
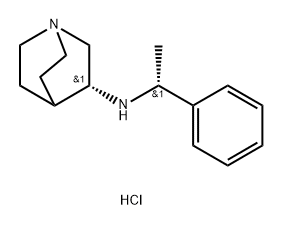 |
Palonosetron Impurity TM1-RR |
1022895-91-5 |
C15H23ClN2 |
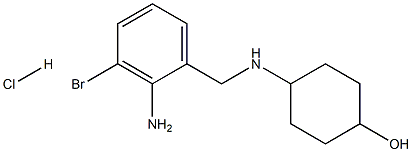 |
(1r,4r)-4-((2-amino-3-bromobenzyl)amino)cyclohexanol hydrochloride |
|
C13H20BrClN2O |
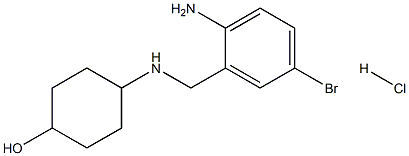 |
(1s,4s)-4-((2-amino-5-bromobenzyl)amino)cyclohexanol hydrochloride |
5579-35-1 |
C13H20BrClN2O |
 |
Moxifloxacin oxide Impurity |
|
|
 |
Edaravone Impurity 18 |
|
|
 |
Linagliptin Impurity 47 |
|
|
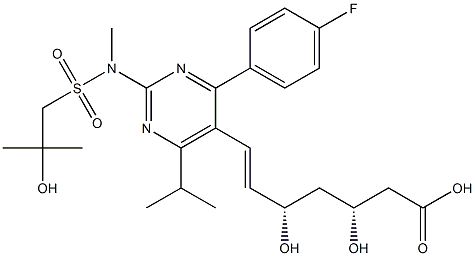 |
(3R,5S,E)-7-(4-(4-fluorophenyl)-2-((2-hydroxy-N,2-dimethylpropyl)sulfonamido)-6-isopropylpyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic acid |
1715120-13-0 |
C25H34FN3O7S |
 |
Cabozantinib impurity 28 |
|
|
 |
Cabozantinib impurity 33 |
|
|
 |
Ondansetron Impurity 2 |
|
|
 |
Rivaroxaban Degradation Impurity C |
|
|
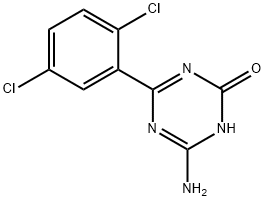 |
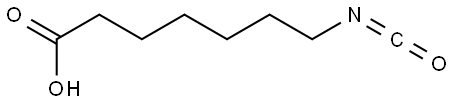
Irsogladine Impurity DQJ |
61382-84-1 |
C9H6Cl2N4O |
 |
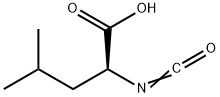
Pregabalin Impurity 7 |
70767-77-0 |
C8H13NO3 |
 |
Pregabalin Impurity 13 |
1089315-68-3 |
C7H11NO3 |
 |
Pralatrexate Impurity 7 |
|
|
 |
Pitavastatin Impurity 39 |
|
|
 |
Dapoxetine impurity 31 |
|
|
 |
Lenvatinib Impurity 36 |
|
|
 |
Sitafloxacin Impurity 19 |
|
|
 |
Febuxostat Impurity 97 |
|
|
 |
Dapoxetine impurity 32 |
|
|
 |
Olprinone Impurity D |
|
|
 |
Canagliflozin Impurity 48 |
|
|
 |
Canagliflozin Impurity 54 |
|
|
 |
Tofacitinib Impurity 105 |
|
|
 |
Cabozantinib impurity Q |
|
|
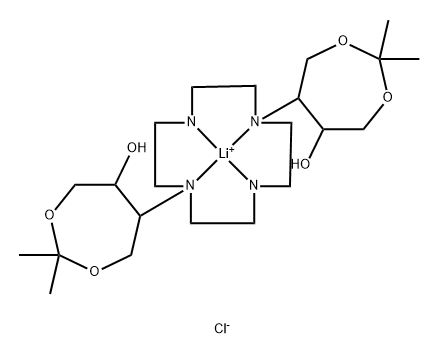 |
Gadobutrol Impurity 1 |
216670-14-3 |
C22H42ClLiN4O6 |
 |
Pemetrexed disodium EP Impurity B |
|
|
 |
Crisaborole Impurity 7 |
|
|
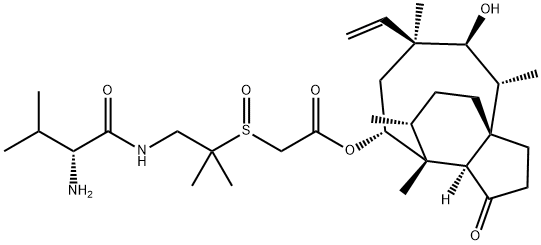 |
Valnemulin EP Impurity A |
1624614-06-7 |
C31H52N2O6S |
 |
Olprinone Impurity F |
|
|
 |
Nicorandil Impurity E |
|
|
 |
Prednicarbate Impurity G |
|
|
 |
Ondansetron IMpurity 6 |
|
|
 |
MeropeneM iMpurity 6 |
|
|