| Structure |
Chemical Name |
CAS |
MF |
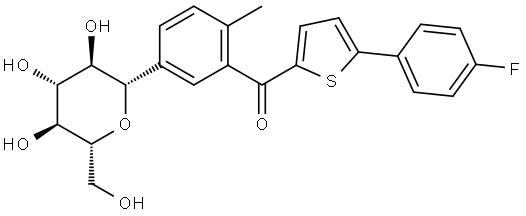 |
Canagliflozin Impurity 17 |
1951467-28-9 |
C24H23FO6S |
 |
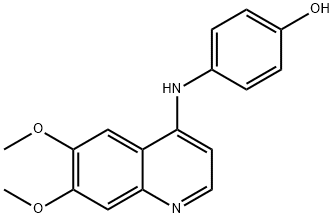
Canagliflozin Impurity 22 |
|
|
 |
Carfilzomib Impurity 3 |
|
|
 |
Dolutegravir Impurity 4 |
|
|
 |
Febuxostat Impurity 20 |
|
|
 |
Lenvatinib Impurity 1 |
|
|
![methyl (41S,12S,13aR)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate](/CAS/20211123/GIF/83508-82-1.gif) |
methyl (41S,12S,13aR)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate |
83508-82-1 |
C21H26N2O3 |
![4-[6-[(8-cyclopentyl-6-ethenyl-7,8-dihydro-5-methyl-7-oxopyrido[2,3-d]pyrimidin-2-yl)amino]-3-pyridinyl]-1-piperazinecarboxylic acid tert-butyl ester](/CAS/20180703/GIF/1941177-45-2.gif) |
4-[6-[(8-cyclopentyl-6-ethenyl-7,8-dihydro-5-methyl-7-oxopyrido[2,3-d]pyrimidin-2-yl)amino]-3-pyridinyl]-1-piperazinecarboxylic acid tert-butyl ester |
1941177-45-2 |
C29H37N7O3 |
 |
Cabozantinib impurity 1 |
748707-58-6 |
C17H16N2O3 |
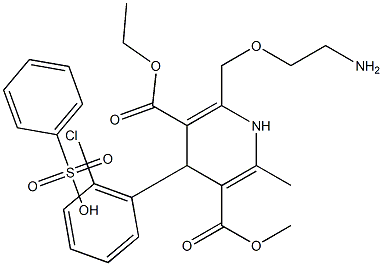 |
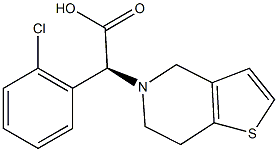
Amlodipine Impurity 19 |
|
C26H31ClN2O8S |
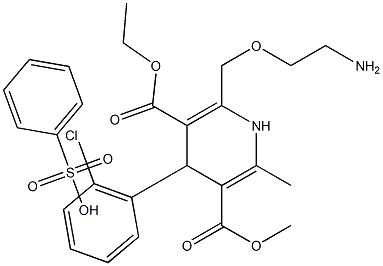 |
Amlodipine Impurity 9 |
90445-05-9 |
C26H31ClN2O8S |
 |
Clopidogrel Impurity 7 |
444728-13-6 |
C15H15ClN2OS |
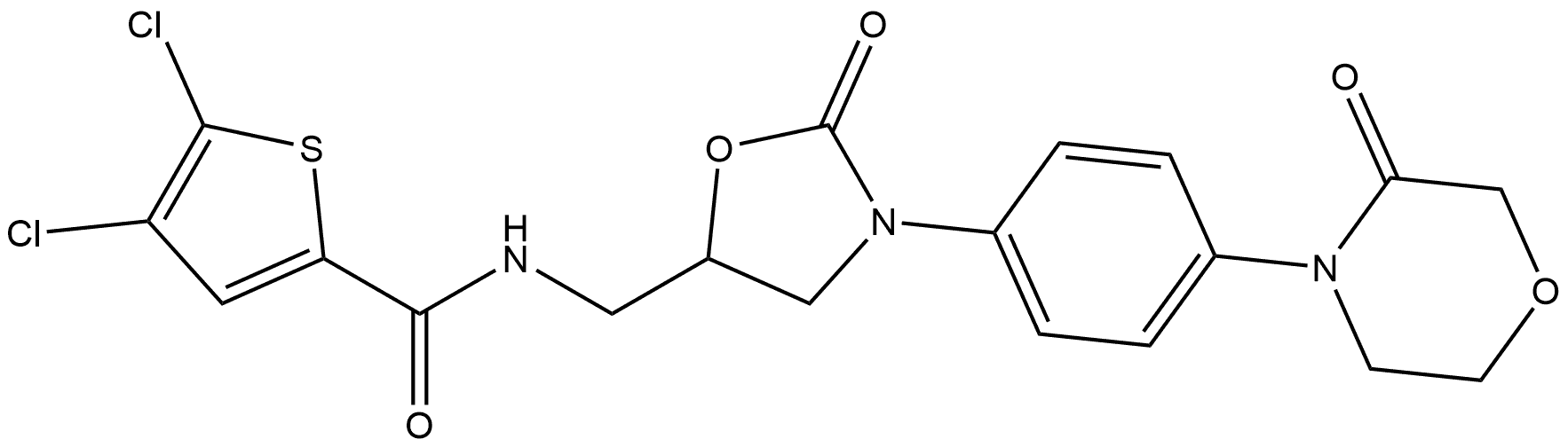 |
Rivaroxaban Impurity 36 |
1770812-39-9 |
C19H17Cl2N3O5S |
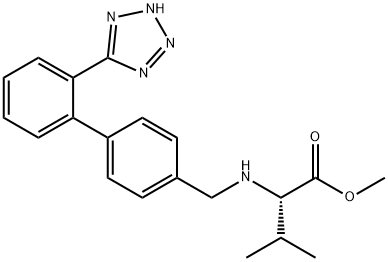 |
(S)-methyl 2-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methylamino)-3-methylbutanoate |
1111177-24-2 |
C20H23N5O2 |
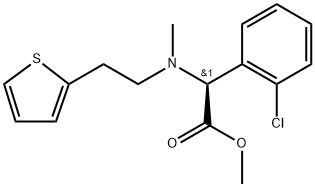 |
Clopidogrel Impurity C |
1346605-15-9 |
C16H18ClNO2S |
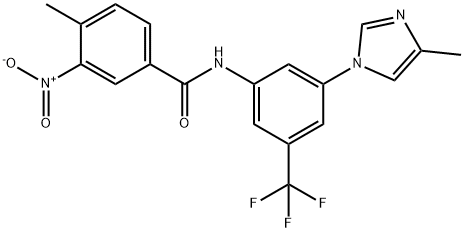 |
Nilotinib genotoxic impurity 2 |
1157857-29-8 |
C19H15F3N4O3 |
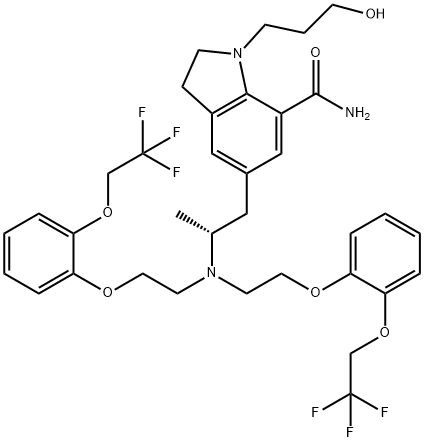 |
NCFJJNFEEPMRAQ-XMMPIXPASA-N |
1453221-45-8 |
C35H41F6N3O6 |
 |
Gliclazide Impurity E |
|
|
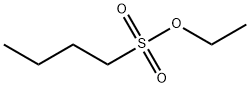 |
Ethyl 1-butanesulfonate |
2374-68-7 |
C6H14O3S |
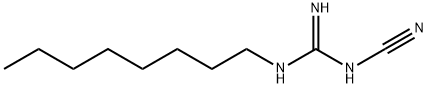 |
OLANEXIDINE intermediate |
60852-95-1 |
C10H20N4 |
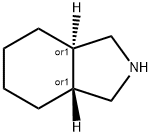 |
1H-Isoindole, octahydro-, trans- |
10479-63-7 |
C8H15N |
![1H-Purine-2,6-dione,8-[2-(3,4-dihydroxyphenyl)ethenyl]-1,3-diethyl-3,7-dihydro-7-methyl-, (E)-(9CI)](/CAS/20180601/GIF/155272-03-0.gif) |
1H-Purine-2,6-dione,8-[2-(3,4-dihydroxyphenyl)ethenyl]-1,3-diethyl-3,7-dihydro-7-methyl-, (E)-(9CI) |
155272-03-0 |
C18H20N4O4 |
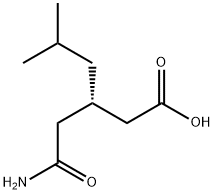 |
Hexanoicacid, 3-(2-amino-2-oxoethyl)-5-methyl-, (3S)- |
181289-34-9 |
C9H17NO3 |
![4-Pyridinecarboxamide, N-[2-(nitrooxy)ethyl]-, mononitrate](/CAS/20180601/GIF/65141-48-2.gif) |
4-Pyridinecarboxamide, N-[2-(nitrooxy)ethyl]-, mononitrate |
65141-48-2 |
C8H10N4O7 |
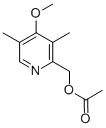 |
2-(ACETOXYMETHYL)-4-METHOXY-3,5-DIMETHYLPYRIDINE |
91219-90-8 |
C11H15NO3 |
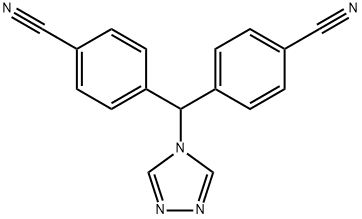 |
4,4'-(4H-1,2,4-TRIAZOL-4-YLMETHYLENE)BIS BENZONITRILE |
112809-52-6 |
C17H11N5 |
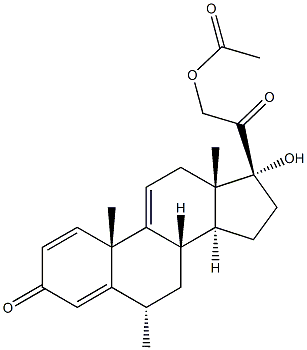 |
Pregna-1,4,9(11)-triene-3,20-dione,21-(acetyloxy)-17-hydroxy-6-methyl-, (6a)- |
93239-37-3 |
C24H30O5 |
 |
Tadalafil EP impurity E |
|
|
![4'-chloro-7H-4,7'-bipyrrolo[2,3-d]pyrimidine](/CAS/20200611/GIF/134965-85-8.gif) |
4'-chloro-7H-4,7'-bipyrrolo[2,3-d]pyrimidine |
134965-85-8 |
C12H7ClN6 |
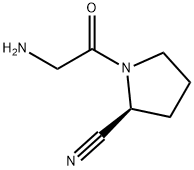 |
(S)-1-(2-Aminoacetyl)-pyrrolidine-2-carbonitrile |
914070-99-8 |
C7H11N3O |
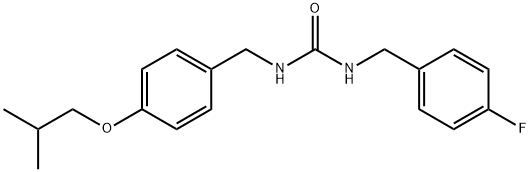 |
1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)urea |
1388858-78-3 |
C19H23FN2O2 |
![1,4,7,10-Tetraazabicyclo[8.2.2]tetradecan-11-one](/CAS/20200401/GIF/220182-11-6.gif) |
1,4,7,10-Tetraazabicyclo[8.2.2]tetradecan-11-one |
220182-11-6 |
C10H20N4O |
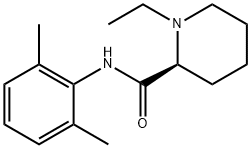 |
Ropivacaine EP Impurity D |
98626-59-6 |
C16H24N2O |
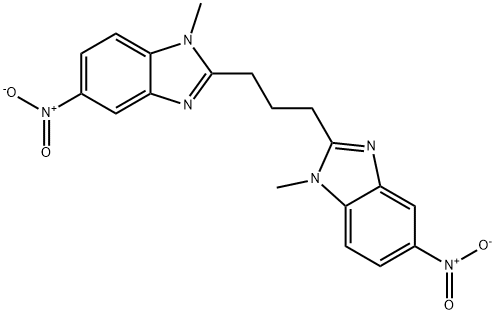 |
Bendamustine Impurity 16 |
914626-65-6 |
C19H18N6O4 |
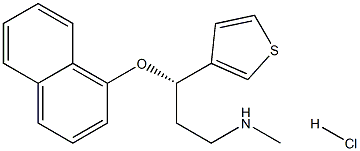 |
(S)-N-methyl-3-(naphthalen-1-yloxy)-3-(thiophen-3-yl)propan-1-amine hydrochloride |
959392-22-4 |
C18H19NOS |
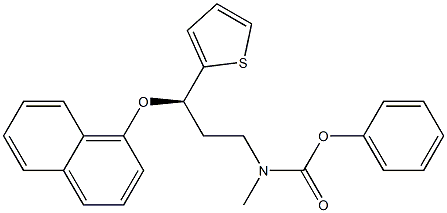 |
phenyl (R)-methyl(3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propyl)carbamate |
603996-87-8 |
C8H11NO2S |
 |
Canagliflozin impurity H |
|
|
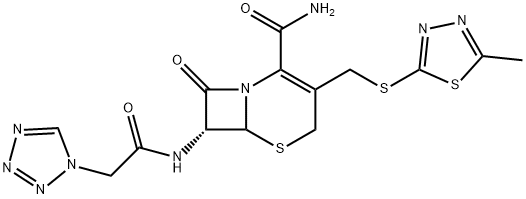 |
Cefazolin Impurity 9 |
1322626-65-2 |
C14H15N9O3S3 |
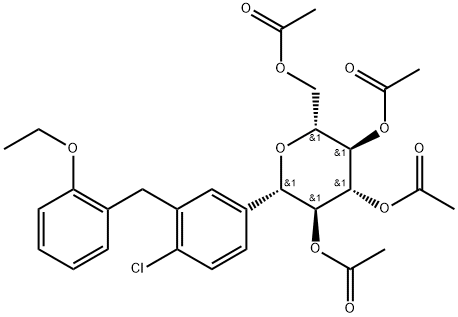 |
Dapagliflozin Impurity 20 |
2040305-09-5 |
C29H33ClO10 |
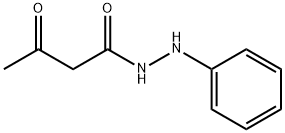 |
Edaravone Impurity 10 |
710307-89-4 |
C10H12N2O2 |
 |
Empagliflozin Impurity 23 |
|
|
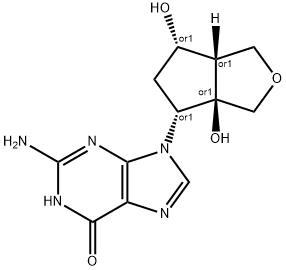 |
Entecavir Impurity 3 |
1391053-94-3 |
C12H15N5O4 |
 |
IBrutinib Impurity C |
|
|
 |
Imatinib impurity 12 |
|
|
 |
Isavuconazole Impurity 20 |
|
|
 |
Isavuconazole Impurity 26 |
|
|
 |
Istradefylline impurity D |
|
|
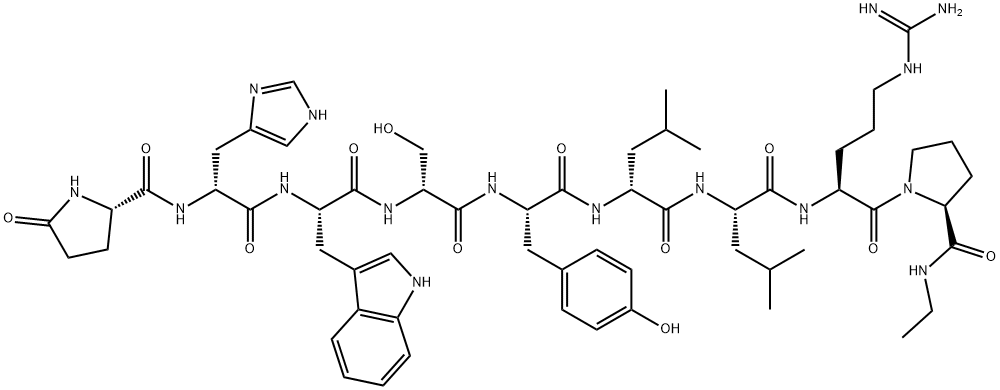 |
Leuprorelin EP Impurity F |
1872435-00-1 |
C59H84N16O12 |
 |
Linagliptin Impurity 40 |
|
|
 |
Minocycline N-Hydroxymethyl Impurity |
1075240-33-3 |
C24H29N3O8 |
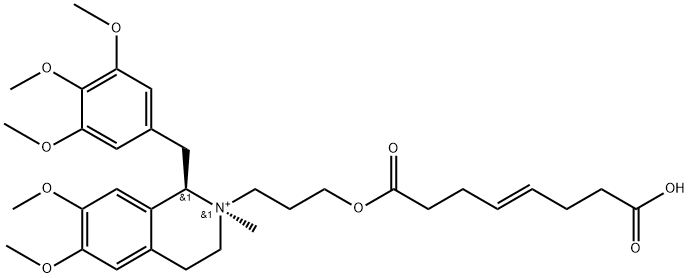 |
Mivacurium Chloride Impurity 3 |
740777-60-0 |
C33H46NO9+ |
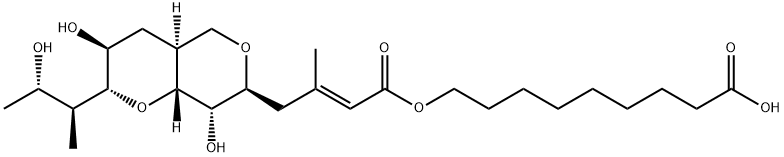 |
Mupirocin EP Impurity E |
71087-96-2 |
C26H44O9 |
 |
Nifuratel Impurity 7 |
|
|
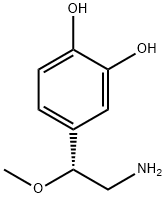 |
Norepinephrine EP Impurity D |
1932110-67-2 |
C9H13NO3 |
 |
RV Salmonella enrichment broth (Pharmacopoeia 2015 edition) |
|
|
 |
Pharmacopoeia experiment |
|
|
 |
Fluconazole impurity I(For Chinese Pharmacopiea) |
|
|
 |
OEM Honey |
|
|
 |
IRAS vials are low alkaline and meet the pharmacopoeia standards of various countries |
|
|
 |
Olaparib Impurity 4 |
|
|
 |
Paclitaxel EP Impurity M |
|
|
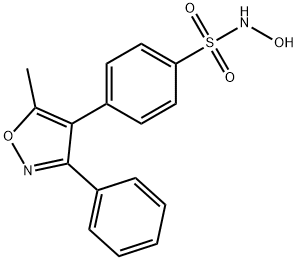 |
Parecoxib Sodium Impurity H |
501093-49-8 |
C16H14N2O4S |
 |
Posaconazole Impurity 10 |
|
|
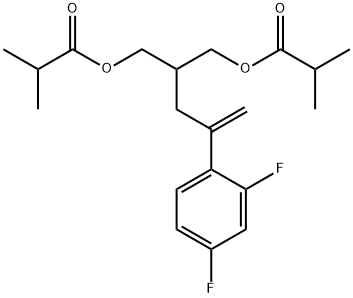 |
Posaconazole Impurity 63 |
208187-15-9 |
C20H26F2O4 |
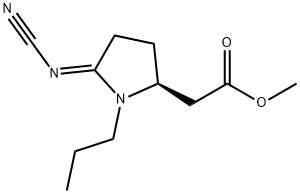 |
(S)-4,5,6,7-Tetrahydro-N2,N6-propionyl-2,6-benzothiazolediaMine |
1373869-91-0 |
C11H17N3O2 |
 |
SofosBuvir impurity 62 |
|
|
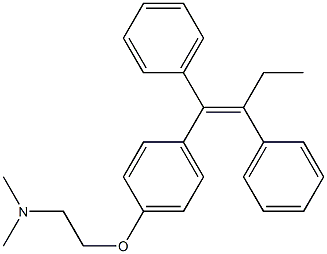 |
Tamoxifen EP Impurity E |
|
C26H29NO |
 |
Tenofovir impurity P |
|
|
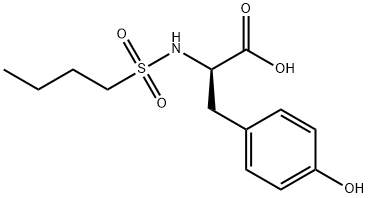 |
Tirofiban Impurity 3 |
1346918-32-8 |
C13H19NO5S |
 |
Trelagliptin Impurity 19 |
|
|
![N-((3R,4R)-1-(2-cyanoacetyl)-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine oxide](/CAS/20180601/GIF/2028267-73-2.gif) |
N-((3R,4R)-1-(2-cyanoacetyl)-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine oxide |
2028267-73-2 |
C16H20N6O2 |
![D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-methoxyphenyl)methyl]phenyl]-, (1S)-](/CAS/20211123/GIF/333359-90-3.gif) |
D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-methoxyphenyl)methyl]phenyl]-, (1S)- |
333359-90-3 |
C20H23ClO6 |
 |
Flucloxacillin Sodium Impurity E |
|
|
 |
Amifostine Impurity 1 |
|
|
 |
Clindamycin Phosphate EP Impurity H |
|
|
![2-Thia-5-azabicyclo[2.2.1]heptane-5-carboxylic acid, 3-oxo-, (4-nitrophenyl)methyl ester, (1S,4S)-](/CAS/20200611/GIF/151072-00-3.gif) |
2-Thia-5-azabicyclo[2.2.1]heptane-5-carboxylic acid, 3-oxo-, (4-nitrophenyl)methyl ester, (1S,4S)- |
151072-00-3 |
C13H12N2O5S |
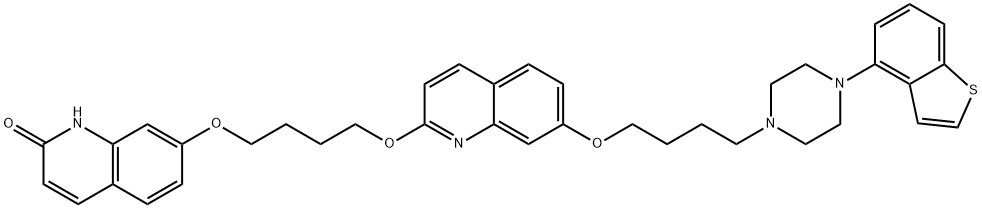 |
Brexpiprazole Impurity |
2116542-21-1 |
C38H40N4O4S |
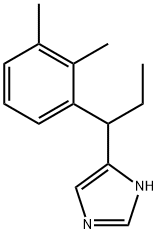 |
4-(1-(2,3-dimethylphenyl)propyl)-1H-imidazole |
86347-62-8 |
C14H18N2 |
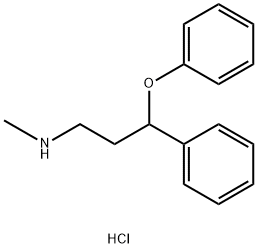 |
Atomoxetine EP Impurity A |
873310-33-9 |
C16H20ClNO |
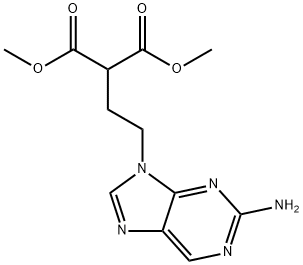 |
Famciclovir Impurity 12 |
122497-20-5 |
C12H15N5O4 |
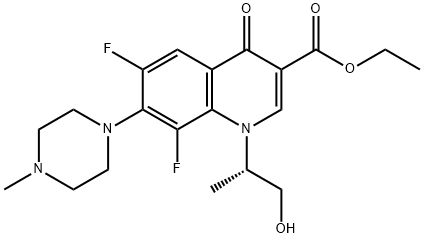 |
Levofloxacin Impurity 22 |
177472-29-6 |
C20H25F2N3O4 |
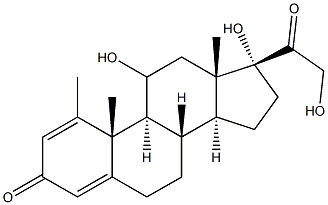 |
Methylprednisolone EP Impurity E |
229019-44-7 |
C21H28O4 |
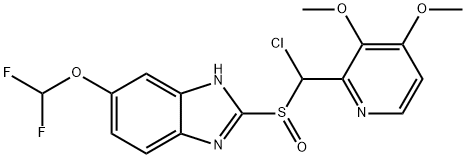 |
Pantoprazole Impurity 1 |
812664-93-0 |
C16H14ClF2N3O4S |
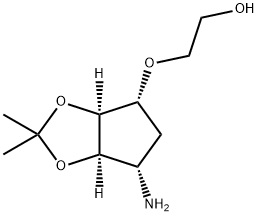 |
Ticagrelor Related Compound 38D-Tartrate |
2165881-71-8 |
C10H19NO4 |
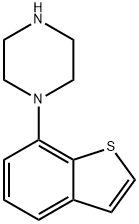 |
1-(1-BENZOTHIOPHEN-7-YL)PIPERAZINE |
105685-06-1 |
C12H14N2S |
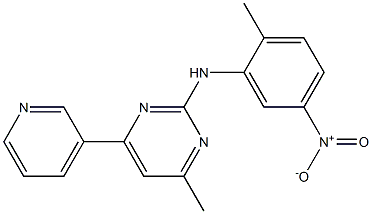 |
4-methyl-N-(2-methyl-5-nitrophenyl)-6-(pyridin-3-yl)pyrimidin-2-amine |
|
C17H15N5O2 |
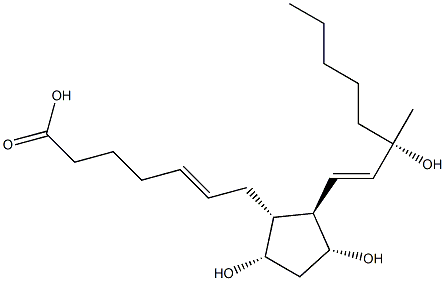 |
trans-Carboprost |
76498-29-8 |
C21H36O5 |
![N-[[2-(1H-Tetrazol-5-Yl)[1,1-Biphenyl]-4-Yl]Methyl]Amine(WXC00176)](/CAS/20180808/GIF/147225-68-1.gif) |
N-[[2-(1H-Tetrazol-5-Yl)[1,1-Biphenyl]-4-Yl]Methyl]Amine(WXC00176) |
147225-68-1 |
C14H13N5 |
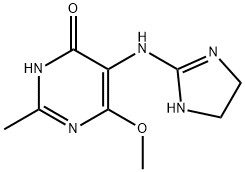 |
MFFANMLJBOSKIX-UHFFFAOYSA-N |
352457-34-2 |
C9H13N5O2 |
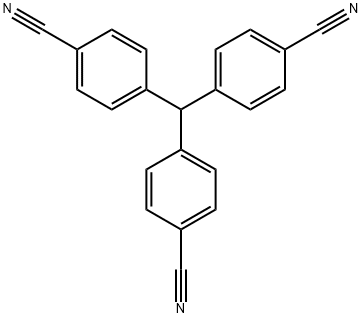 |
4,4',4''-Methylidenetrisbenzonitrile |
113402-31-6 |
C22H13N3 |
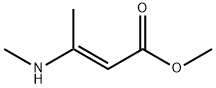 |
methyl (2E)-3-(methylamino)but-2-enoate |
21759-69-3 |
C6H11NO2 |
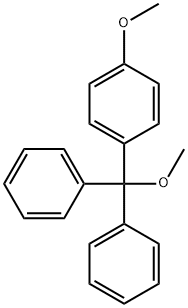 |
Entecavir Impurity 5 |
84868-56-4 |
C21H20O2 |
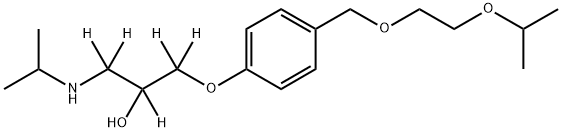 |
Bisoprolol-d5 |
1189881-87-5 |
C18H31NO4 |
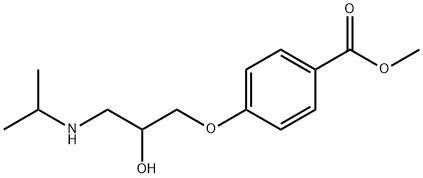 |
4-(2-HYDROXY-3-ISOPROPYLAMINO-PROPOXY)-BENZOIC ACID METHYL ESTER |
33947-97-6 |
C14H21NO4 |
![3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-2-[1-[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]pentan-2-yl]-1,3-diazaspiro[4.4]non-1-en-4-one](/CAS/20180601/GIF/1346598-52-4.gif) |
3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-2-[1-[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]pentan-2-yl]-1,3-diazaspiro[4.4]non-1-en-4-one |
1346598-52-4 |
C39H38N10O |
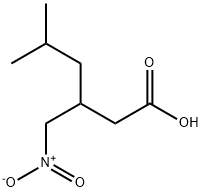 |
5-METHYL-3-NITROMETHYL-HEXANOIC ACID |
181289-21-4 |
C8H15NO4 |
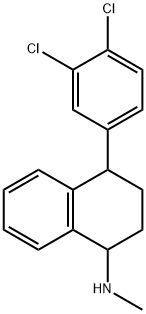 |
4(3,4-DICHLOROPHENYL)1,2,3,4-TETRAHYDRO-N-METHYL-1-NAPHTHALENE AMINE RACEMATE |
140631-53-4 |
C17H17Cl2N |
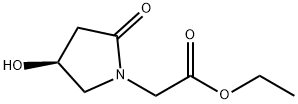 |
(S)-ethyl 2-(4-hydroxy-2-oxopyrrolidin-1-yl)acetate |
870695-39-9 |
C8H13NO4 |
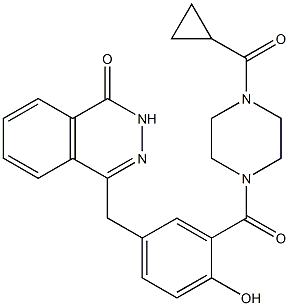 |
4-(3-(4-(cyclopropanecarbonyl)piperazine-1-carbonyl)-4-hydroxybenzyl)phthalazin-1(2H)-one |
|
C24H24N4O4 |
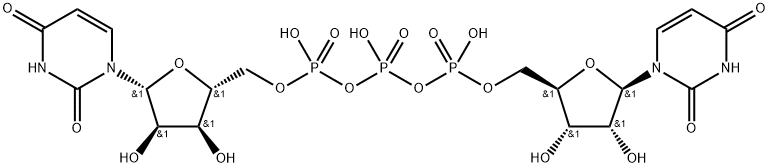 |
Diquafosol Impurity 2 |
63785-59-1 |
C18H25N4O20P3 |