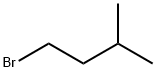
????????? C??? ??, ??, ??
??? ??
clear colourless liquid
??
1-Bromo-3-methylbutane was used in the synthesis of 1-(3-methylbutyl)pyrrole and in the production of pentyl peroxy radical by direct photolysis production method. It was also used as extraction solvent for determination of polycyclic aromatic hydrocarbons in water samples using liquid-liquid microextraction combined with gas chromatography-mass spectrometry. It is also used as an alkyl halide and organic synthesis reagent.
?? ??
A colorless liquid. Flash point 21°F. Slightly soluble in water and denser than water. Vapors are heavier than air.
??? ?? ??
Highly flammable. Slightly soluble in water and denser than water.
?? ???
Halogenated aliphatic compounds, such as 1-Bromo-3-methylbutane, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Gives toxic fumes of bromine when burned.
????
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
????
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by
intraperitoneal route. Flammable liquid.
Dangerous fire hazard when exposed to heat
or flame. When heated to decomposition it
emits toxic fumes of Br-. See also
BROMIDES.
Purification Methods
Shake the bromide with conc H2SO4, wash with water, dry with K2CO3 and fractionally distil it. [Beilstein 1 IV 378.]
????????? ?? ?? ? ???
???
?? ??