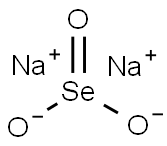?? ?????
|
|
?? ????? ??
- ???
- >350 °C (lit.)
- ??
- 3.1[at 20℃]
- ???
- 0.133Pa at 20℃
- ?? ??
- Store at RT.
- ???
- ?(?? ???)
- ??? ??
- ??
- ??
- ???
- ??
- ?? ??
- ???? ??
- synthetic (inorganic)
- ???
- 950g/L(20℃)
- ??
- Hygroscopic
- Merck
- 14,8673
- ?? ??
- ACGIH: TWA 0.2 mg/m3
NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3
- InChIKey
- BVTBRVFYZUCAKH-UHFFFAOYSA-L
- CAS ??????
- 10102-18-8(CAS DataBase Reference)
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | T+,N,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 23-28-31-43-51/53-36/37/38 | ||
| ????? | 28-36/37-45-61-28A-36-26 | ||
| ????(UN No.) | UN 2630 6.1/PG 1 | ||
| WGK ?? | 2 | ||
| RTECS ?? | VS7350000 | ||
| TSCA | Yes | ||
| ?? ?? | 6.1 | ||
| ???? | I | ||
| HS ?? | 28429010 | ||
| ?? ?? ??? | 10102-18-8(Hazardous Substances Data) | ||
| ?? | LD50 orally in rats: 7 mg/kg (Cummins, Kimura) | ||
| ???? ?? | KE-31605 | ||
| ?????? ??? | 97-1-134 | ||
| ?? ? ???? | ????: ????; ???(??)????: ??? ?? ? ???? ?????? 1% ?? ??? ??? |
?? ????? C??? ??, ??, ??
????
? (1) ??? : ? ?? 0.5g? ??? ? 1mL? ?? ? ???? 2mL? ??? ?, ??? ???? ??? ??.
??(2) ??? : ? ?? 0.5g? ??????? ?? ??? ?, ?? 0.05mg ????? ??(0.01% ??).
??(3) ??? : ? ?? 0.2g? ? 3mL? ??? ????????? ??? 50mL? ? ?? ?????? ??. ??, ??????? 2mL ? ? ?? 0.2g? ????????? ??? 50mL? ? ?? ????? ??, ???????? 50mL? ??????? ??. ????, ??? ? ?????? 10?? ?? ??? ???? ??? ???? ?? ?? ??????? ?????? ??? ???? ?? 410nm?? ???? ????? ? ????? ???? ???? ????? ???? ?? ??(0.01% ??).
???? ?
???????????? : ?????? 600mg? ?? ?? ??(2→3) 600mL? ?? ? ??(2→3)? ??? 1,000mL? ??.
??????????? : ???? 163mg? ?? ?? 100mL? ??, ?? 10mL? ?? ?? ??? 1,000mL? ??(? ? 1mL? ???(NO3) 0.01mg ??).
??(4) ???? ? ??? : ? ?? 0.5g? ????? 20mg? ?? 10mL? ?? ? ??? ?? ?? ??? ??? ?????. ???? ?? 1mL? ?? ? ?? ???? ????. ??? ???? ??? ? 15mL? ?? 1mL? ??? ??????? ?? ??? ?, ?? 0.15mg?? ??? ?? ????? ??(0.03% ??).
????
? ? ?? 50mg? 0.1N ?? 5mL? ??? ?? ? ?? ?????? 50mg? ??? ???~????? ??? ????.
???
? ? ??? ?????? ? 100mg? ???? ?? ??? ?? ??????? ?? ?? ? 12mL? ?? ??? ?? ??? ????. ???? ? 15?? ??? ??? ???? ?? ?? ???? 80mL? ?? ??? ??? ??? ????. ? ??? ??? ??? ??? ??? ????? ???? ???? ?? 50mL ????? ??? ?? ??????? ???????(4→200)?? ???? ?? ? ? ???????(4→200)? ??? 50mL? ??. ? ??? ??? ??? ??? ??? ???? ?????? ??. ???? ? ? ????? ???????(4→200)·????? ??(20 : 1)? ????? ?? ??? ?????? ??????? ?? ?????????????? ?? ???? ???? ????, ????? ???? ?? C(mg/mL)? ??? ?? ???? ?? ???????? ??(%)? ???.
?
? D : ????
?
?????? : ??? ? 1.0g? ??? ?? ?? ???? ??? ???? ???? ?? ? 2mL? ??? ??????. ? ??? 3? ????? 3N ??? ??? 1L? ? ?? ?????? ??. ?? ? ? 10mL? ???? ??? ?? ??? 100mL? ??, ? ? 5, 10 ? 25mL? 100mL ????? ?? ??? ?? ???? 5mL?? ?? 15?? ??? ??? ???? ?? ?? ???????(4→200)? ??? 100mL? ??. ? ? 1mL? 0.005, 0.01 ? 0.025mg? ???? ????.
?
??????
???????? ?????????
????????? 196nm
????????? ?? ?
????????? ????