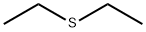?????? C??? ??, ??, ??
??? ??
colourless liquid
??
Reported found in cabbage, sauerkraut, mustard, egg, chicken, beef, pork, beer (0.0002 to 0.0017 ppm), grape
brandy (cognac, armagnac, weinbrand).
??
Solvent for Anhydrous mineral salts; in plating baths for coating metals with gold or silver.
??
ChEBI: An ethyl sulfide compound having two ethyl groups attached to a sulfur atom.
?? ??
A colorless oily liquid with a garlic-like odor. Less dense than water. Flash point 20°F. Vapors heavier than air. May irritate skin and eyes. Used to make other chemicals.
??? ?? ??
Highly flammable. Slightly soluble in water.
?? ???
Organosulfides, such as 1,1'-Thiobisethane, are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid.
????
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
????
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by
ingestion. A skin and eye irritant. A very
dangerous fire hazard when exposed to heat,
flame, or sparks; can react vigorously with
oxidizers. Reacts with water, steam, acids, or
acid fumes to produce toxic and flammable
vapors. To fight fire, use water spray or
mist, dry chemical, CO2, foam. When heated
to decomposition it yields highly toxic
fumes of SOx. See also SULFIDES.
Purification Methods
Wash the sulfide with aqueous 5% NaOH, then water, dry with CaCl2 and distil it from sodium. It can also be dried with MgSO4 or silica gel. Alternative purification is via the Hg(II) chloride complex [(Et)2S.2HgCl2] (see dimethyl sulfide). [Beilstein 1 IV 1394.]
?????? ?? ?? ? ???
???
?? ??