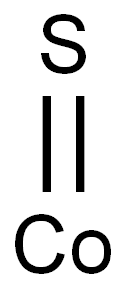??? ????
??? ???? ??
???
>1116°C
??
5,45 g/cm3
???
0Pa at 20℃
RTECS ??
GG3325000
???
insoluble in H2O; soluble in acid solutions
??? ??
??
Specific Gravity
5.45
??
???
???
?? ???.
??
moisture sensitive
Crystal Structure
NiAs type
crystal system
Six sides
Space group
P63/mmc
Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.337 0.337 0.516 90 90 120 0.0508
?? ??
ACGIH: TWA 0.02 mg/m3
CAS ??????
1317-42-6(CAS DataBase Reference)
EPA
Cobalt(II) sulfide (1317-42-6)
??
?? ? ?? ??
?? ? ???? ?? (GHS)
????(GHS):
?? ?:
??·?? ??:
??
??·?? ??
?? ??
??
?? ?
?? ??
P- ??
H317 ????? ?? ??? ??? ? ??
?? ??? ??
?? 1
??
P261, P272, P280, P302+P352,P333+P313, P321, P363, P501
H400 ????? ?? ???
?? ????? ?? - ??
?? 1
??
P273, P391, P501
H410 ??? ??? ?? ????? ?? ???
?? ????? ?? - ??
?? 1
??
P273, P391, P501
??????:
P261 ??·?·??·???·??·...·????? ??? ????.
P321 (…) ??? ???.
P333+P313 ????? ?? ??? ???? ???? ??·??? ????.
NFPA 704
??? ???? C??? ??, ??, ??
??? ??
Brown to raddish powder
??
Cobalt Sulfideis used as a catalyst for hydrogenation or hydrodesulfurization reactions. Cobalt sulfide is found in nature as the mineral sycoporite.
Safety Profile
Suspected carcinogen
with experimental tumorigenic data.
Mutation data reported. If dried at 300’C it
igmtes spontaneously in air. See also
COBALT COMPOUNDS and SULFIDES.
??? ???? ?? ?? ? ???
???
?? ??
??? ???? ?? ??
???( 82)?? ??
China 42
Europe 1
France 1
Germany 3
India 7
Japan 2
United Kingdom 5
United States 21
Global 82