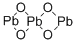????(Ⅳ)
|
|
????(Ⅳ) ??
- ???
- 500 °C
- ?? ?
- 800°C
- ??
- 9,1 g/cm3
- ???
- 10 mm Hg ( 0 °C)
- ???
- insoluble in H2O, ethanol; soluble in hotHCl
- ??? ??
- ?? ??
- ??
- ??? ??
- ??
- ????
- ???
- ??, ???, ?? ? ?????? ?????. ?? ???? ???.
- Merck
- 14,5425
- crystal system
- square
- Space group
- P42/mbc
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.882 0.882 0.659 90 90 90 0.5127
- ?? ??
- ACGIH: TWA 0.05 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3
- Dielectric constant
- 25.9(0.0℃)
- ???
- ????. ???? ???? ??? ? ??.
- InChIKey
- XMFOQHDPRMAJNU-UHFFFAOYSA-N
- CAS ??????
- 1314-41-6(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | O,T,N | ||
|---|---|---|---|
| ?? ???? ?? | 61-8-20/22-33-50/53-62-48/23/25-40 | ||
| ????? | 53-45-60-61-36/37 | ||
| ????(UN No.) | UN 1479 5.1/PG 2 | ||
| WGK ?? | 3 | ||
| RTECS ?? | OG5425000 | ||
| TSCA | Yes | ||
| ?? ?? | 5.1 | ||
| HS ?? | 28249010 | ||
| ?? ?? ??? | 1314-41-6(Hazardous Substances Data) | ||
| ?? | LD50 i.p. in rats: 45 mg Pb/100 g (Lead, 1972) | ||
| ???? ?? | KE-27408 | ||
| ?????? ??? | ??1-105 |
????(Ⅳ) C??? ??, ??, ??
??
Lead oxide, a range of products that is formed by the oxidation of Lead in the forms of liquid and solid. Lead oxides are basically an oxide’s family varying in color (grey/green, red, and yellow), in degree of oxidation (PbO, Pb3O4, PbO2) and in crystal structure (in forms of PbO, orthogonal and tetragonal).Lead oxide is a term that can be either Lead monoxide or litharge Lead tetroxide or Red Lead or Gray or Black oxide which is a mixture of 30 percent metallic Lead and 70 percent Lead monoxide. Black Lead is made for specific use in Lead acid storage batteries manufacturing. Due to large use in the Lead acid battery industry, Lead monoxide is one of the most important compounds of Lead, based on volume. Due to its electrical and electronic properties, litharge is also used in various components for different types of use like capacitors, electro photographic plates, and Video tubes, even in ferromagnetic and ferroelectric materials. Their wide range of chemical and physical properties, Lead oxides have been know and used worldwide since before the ancient Romans.
??? ??
red to orange powder??
Plasters and ointments; manufacture of colorless glass; glaze for faience; flux for porcelain painting, protective paint for iron and steel; oil-color for ship paints, varnishes; coloring rubber; cement for glass, gas and steam pipes; storage batteries; pencils for writing on glass; manufacture of lead peroxide, matches.?? ??
Lead(IV) oxide, Pb2O, is obtained by action of chlorine on alkaline solutions of lead(II) oxide or acetate. The reaction is Pb(OH)3-+ ClO- → PbO2 + Cl- + OH- + H2O·PbO2 can also be produced on a lead or platinum anode by electrolysis in acidic solution.??
Natural red oxide of lead.World Health Organization (WHO)
Lead oxides and other lead salts were formerly available in topical preparations which had soothing astringent properties. The toxicity of lead salts by inhalation, ingestion and percutaneous absorption is now conclusively established and the medicinal use of preparations containing lead salts is no longer permitted in many countries.?? ??
Lead oxide is used quite extensively in optical glass, electrical glass, and tableware. It increases the density and refractive index of glass. In addition, it can be cut more easily than other glasses and has superior brilliance, both of which make it good for cut glass.Lead glasses may be formulated with a wide variety of electrical and acid-resisting characteristics; desirable properties, such as weather resistance, electrical resistivity, etc., will depend upon the total composition of the glass.
????(Ⅳ) ?? ?? ? ???
???
?? ??
????(Ⅳ) ?? ??
???( 266)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085; +8615531157085 |
abby@chuanghaibio.com | China | 8807 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12789 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29856 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5854 | 58 |
| Hebei Runbin Biotechnology Co. LTD | 13180553332 |
2179877681@qq.com | CHINA | 990 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |