1,3-Bis (2,6-diisopropylphenyl) imidazolium chloride Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
White to Light yellow to Light orange powder to crystal. soluble in methanol.
Verwenden
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride is also used in organic synthesis, as well as a pharmaceutical intermediate. 1,3-Bis(2,6-diisopropylphenyl)imidazolium Chloride is used as a reagent in the synthesis of NHC Copper(I) complexes bearing dipyridylamine ligands which exhibit interesting luminescent properties and are potential candidates for organic light-emitting diode applications. 1,3-Bis(2,6-diisopropylphenyl)imidazolium Chloride is also used as a reagent in the synthesis of 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic Acid Methyl Ester (D476595); the methyl ester derivative of the drug Vadimezan (V084950).
Application
1,3-Bis(2,6-Diisopropylphenyl)imidazolium chloride is also used as a reagent in the synthesis of 5,6-Dimethyl-9-Oxo-9H-xanthene-4-acetic acid methyl ester; the methyl ester derivative of the drug Vadimezan. Moreover, it is an imidazolium salt that is active against all stages of Trypanosoma Cruzi and may represent a promising candidate for treatment of Chagas disease.
Reaktionen
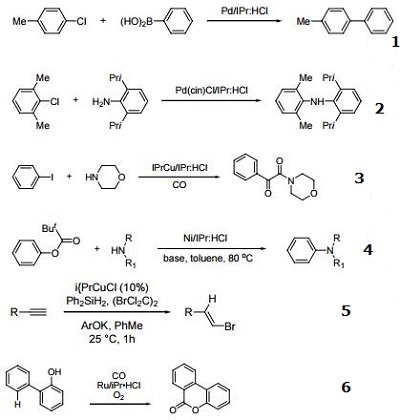
Precursor to Pd catalysts used in C-N and C-C coupling reactions.
Ligand used in double carbonylation reactions.
Precursor to Ni catalysts used in C-N coupling reactions.
Precursor to Cu catalysts used in copper hydride reactions.
Ligand used in Ru-catalyzed carbonylative C-H cyclization of 2-aryl phenols.
Sicherheit(Safety)
H300 (37.84%): Fatal if swallowed [Danger Acute toxicity, oral]
H315 (62.16%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (37.84%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H318 (37.84%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H319 (62.16%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H400 (37.84%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410 (37.84%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
Einzelnachweise
Suzuki-Miyaura ReactionEsterification of α-Halo-α,β-unsaturated Aldehydes by Using a Carbene CatalystCycloaddition of CO2 to Epoxides Catalyzed by N-Heterocyclic Carbene (NHC)–ZnBr2 System under Mild Conditions[1] Yan C, et al. An efficient and recyclable iron(III)-containing imidazolium salt catalyst for cross-coupling of aryl Grignard reagents with alkyl halides. Science Bulletin, 2012; 57: 1953–1958.
[2] Hans M, et al. Efficient synthetic protocols for the preparation of common N-heterocyclic carbene precursors. Beilstein Journal of Organic Chemistry, 2015; 11: 2318–2325.
1,3-Bis (2,6-diisopropylphenyl) imidazolium chloride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

