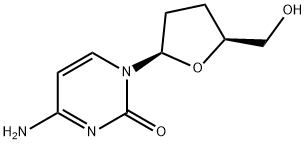
Zalcitabine
|
|
Zalcitabine Eigenschaften
- Schmelzpunkt:
- 217-218 °C(lit.)
- Siedepunkt:
- 350.9°C (rough estimate)
- alpha
- D25 +81° (c = 0.635 in water)
- Dichte
- 1.2605 (rough estimate)
- Brechungsindex
- 78 ° (C=0.5, H2O)
- storage temp.
- Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
- L?slichkeit
- DMSO (Slightly, Heated), Methanol (Slightly), Water (Slightly, Sonicated)
- Aggregatzustand
- powder
- pka
- 14.44±0.10(Predicted)
- Farbe
- colorless
- Wasserl?slichkeit
- 5-10 g/100 mL at 19 ºC
- Merck
- 14,10109
- BRN
- 654956
- Stabilit?t:
- Stable. Combustible. Incompatible with strong oxidizing agents.
- InChIKey
- WREGKURFCTUGRC-POYBYMJQSA-N
- CAS Datenbank
- 7481-89-2(CAS DataBase Reference)
- IARC
- 2B (Vol. 76) 2000
- EPA chemische Informationen
- Cytidine, 2',3'-dideoxy- (7481-89-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn,C | ||
|---|---|---|---|
| R-S?tze: | 40-36/37-34 | ||
| S-S?tze: | 22-36-45-36/37/39-27-26 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | HA3870000 | ||
| F | 10-23 | ||
| HS Code | 2934990002 | ||
| Giftige Stoffe Daten | 7481-89-2(Hazardous Substances Data) |
| Bildanzeige (GHS) |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Zalcitabine Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R40:Verdacht auf krebserzeugende Wirkung.R36/37:Reizt die Augen und die Atmungsorgane.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S27:Beschmutzte, getr?nkte Kleidung sofort ausziehen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Beschreibung
Zalcitabine is an orally active dideoxynucleoside andog for combination use with zidovudine in advanced HIV infection and also as monotherapy for AIDS patients who cannot tolerate or have not responded to zidovudine. It has a similar mechanism of action (inhibition of reverse transcriptase) to didanosine. Like didanosine, its side effect profile includes peripheral neuropathy. Unlike zidovudine, zalcitabine does not cause bone marrow suppression.Chemische Eigenschaften
White to Off-White Cyrstalline PowderDefinition
ChEBI: A pyrimidine 2',3'-dideoxyribonucleoside compound having cytosine as the nucleobase.Indications
Zalcitabine (ddC, Hivid) is a cytidine analogue active against HIV-1, HIV-2, and hepatitis B virus. It is used for the treatment of HIV infection in adults and asymptomatic children as part of a multidrug regimen. It may be less effective than the other nucleoside inhibitors and is used less frequently.Allgemeine Beschreibung
Zalcitabine, 2',3'-dideoxycytidine or ddCyd, is an analog ofcytosine that demonstrates activity against HIV-1 and HIV-2,including strains resistant to AZT. The potency (in peripheralblood mononuclear cells) is similar to that of AZT, but thedrug is more active in populations of monocytes andmacrophages as well as in resting cells.The oral bioavailability of zalcitabine is over 80% in adultsand less in children.The major dose-limiting side effect isperipheral neuropathy, characterized by pain, paresthesias,and hypesthesia, beginning in the distal lower extremities.These side effects are typically evident after several months oftherapy with zalcitabine. A potentially fatal pancreatitis is anothertoxic effect of treatment with ddC. The drug has beenapproved for the treatment of HIV infection in adults with advanceddisease who are intolerant to AZT or who have diseaseprogression while receiving AZT. ddC is combined with AZTfor the treatment of advanced HIV infection.
Air & Water Reaktionen
Water soluble.Reaktivit?t anzeigen
Zalcitabine may be sensitive to prolonged exposure to light.Brandgefahr
Flash point data for Zalcitabine are not available; however, Zalcitabine is probably combustible.Pharmakologie
Peripheral neuropathy occurs in up to 50% of patients taking zalcitabine. Stomatitis, esophageal ulceration, hepatotoxicity, rash, and pancreatitis may occur. Zalcitabine should be used with caution in individuals with a history of pancreatitis, liver disease, or alcohol abuse. Dosage adjustment is necessary for individuals with renal impairment. Zalcitabine should not be used in combination with didanosine, lamivudine, or stavudine.Pharmakokinetik
Zalcitabine (ddC) is a useful alternate drug to ZDV and is given in combination with ZDV when CD4 cell counts fall to less than 300 cells/mm3 . Monotherapy with ddC is more active than ZDV. Its oral bioavailability is 87%, and its plasma half-life is approximately 1 hour. In low doses (0.005 mg/kg every 4 hours), ddC produces sustained decrease in p24 antigen level and increase in CD4 cell counts. The CSF fluid/plasma ratio of ddC is 0.2. Following oral administration, bioavailability of ddC is less than 80%, which is further reduced when taken with food. The mean maximum plasma concentration of the drug also is reduced from 25.2 to 15.5 ng/mL when the drug was taken with food.Nebenwirkungen
It has side effects, such as stomatitis, rash, fever, malaise, arthritis, and arthralgia.Stoffwechsel
Dideoxyuridine is the major metabolite in urine and feces. The drug penetrates the blood-brain barrier. The major toxicity of ddC is peripheral neuropathy, in which case it should be discontinued. In some cases, pancreatitis occurs when given alone or in combination with ZDV."Zalcitabine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Acetylbromid
Palladium
Wasserstoff
Cytidin
Ammoniak, wasserfrei
Edetinsure
2-Acetoxy-2-methylpropionyl bromide
Benzyltrimethylammoniumhydroxid
Palladiumdihydroxid
Essigs?ureanhydrid
4-Acetyl-1-(β-D-ribofuranosyl)cytosin
Zink
POLY(4-VINYLPYRIDINE)
ZALCITABINE RELATED COMPOUND A (50 MG) (2',3'-DIDEHYDRO-2',3'-DIDEOXYCYTIDINE)
(S)-5-Hydroxymethyldihydrofuran-2-one
Ammoniak, wssrige Lsung
Downstream Produkte
Zalcitabine Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 360)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12832 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5857 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18148 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 970 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29879 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 |
figo.gao@foxmail.com | China | 8497 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Nanjing Baifuli Technology Co., Ltd. | +86-15335185688 |
sales@unisyn.cn | CHINA | 332 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19553 | 58 |
7481-89-2()Verwandte Suche:
Cytarabin
2-(β-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-on
5-Fluor-2'-desoxyuridin
Cytidine-5'-triphosphate disodium salt dihydrate
Cytidin
Flucytosin
(±)-N-(Tetrahydro-2-oxothien-3-yl)acetamid
Dideoxyinosine
Cytosin
5-Fluorocytidine
1-β-D-Arabinofuranosylcytosinhydrochlorid
1-[4-[[2-O-(6-Desoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(3-hydroxy-4-methoxyphenyl)propan-1-on
10,11-Dihydro-α-methyl-10-oxodibenzo[b,f]thiepin-2-essigsure
DNA, SINGLE STRANDED, IMM. ON CELLULOSE, FROM CALF THYMUS*
Cytidin-3'-monophosphat
N-Benzoyl-5'-O-(p,p'-dimethoxytrityl)-2'-desoxycytidin
4-Acetyl-1-(β-D-ribofuranosyl)cytosin
2'-Desoxy-5-methylcytidin
- 4-AMINO-1-[(2R,5S)-5-(HYDROXYMETHYL)OXOLAN-2-YL]PYRIMIDIN-2-ONE
- ZALCITABINE
- 1-(2',3'-DIDEOXY-BETA-RIBOFURANOSYL)CYTOSINE
- 2',3'-Dideoxy-D-cytidine
- 2'',3''-DIDEOXYCYTIDINE(ZALCITABINE)
- Cytidine, 2',3'-dideoxy- (8CI, 9CI)
- D 2C
- NSC 606170
- Ro 24-2027/000
- BETA-D-2',3'-DIDEOXYCYTIDINE
- 2’,3’-dideoxy-cytidin
- ZALCITABINE [2'',3''-DIDEOXYCYTIDINE]
- 23Dideoxycytidine, Dideoxycytidine, ddCyd, Hivid
- ddCyd
- I-livid
- 2'', 3''-DIDEOXYCYTIDINE (ZALCITABINE: DDC)
- 2',3'-Dideoxycytidine, 98+%
- 1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine
- 2',3'-ddC
- 2',3'-dideoxy cytidi
- Zalcitabine(2'-3'-dideoxycytidine, ddC)
- 3'-Dideoxycytidine
- Zalcitabine (200 mg)
- ddC 1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine Zalcitabine
- 4-AMino-1-((2R,5S)-5-(hydroxyMethyl)tetrahydrofuran-2-yl)pyriMidin-2(1H)-one
- 4-Amino-1-(2,3-dideoxy-β-D-ribofuranosyl)pyrimidine-2(1H)-one
- 2',3'-DIDEOXYCYTIDINE extapure
- 2',3'-Dideoxycytidine, 99.0%
- DDC;DIDEOXYCYTIDIN
- Dideoxycytidin
- N-[2-(2-bromo-4,5-dimethoxyphenyl)ethyl]carbamic acid methyl ester
- DDC(ZALCITABINE)
- Cytidine, 2',3'-dideoxy-
- 2’,3’-Dideoxycytidine, ddC, Zalcitabine
- Human DDC Protein, His Tag
- Zalcitabine USP/EP/BP
- 2,3-DideoxycytidineQ: What is 2,3-Dideoxycytidine Q: What is the CAS Number of 2,3-Dideoxycytidine Q: What is the storage condition of 2,3-Dideoxycytidine Q: What are the applications of 2,3-Dideoxycytidine
- 2,3-Dideoxycytidine (ddC) extrapure, 98%
- Zalcitabine (1724306)
- 2',3'-DIDEOXYCYTIDINE
- Hivid
- dideoxycytidine
- DDC
- 2',3'-Didecxycvtidine
- Zalcitabine (200 mg) (DISCONTINUED)
- 2', 3'-Dideoxycytidine
- 2',3'-ddC
- 2',3'-Dideoxycytidine/Zalcitabine
- 2',3'-Didecxycvtidine
- 2'',3''-Dideoxycytidine (ddC), nucleoside reverse transcriptase inhibitor (NRTI)
- 7481-89-2
- C9H13N3O3
- Immune Cell Signaling and Blood
- Immune System Regulation
- Nucleoside Analogs
- Nucleosides, Nucleotides, Oligonucleotides
- Biochemicals and Reagents
- AIDS and Viral Research Reagents