Poly(methylhydrosiloxane) Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R10:Entzündlich.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).
S16:Von Zündquellen fernhalten - Nicht rauchen.
Beschreibung
Poly(methylhydrosiloxane) is a reducing agent. For example, it can
be used for the reduction of esters to alcohols as well as the
reduction of aldehydes and ketone. It can also be used for the
reduction of phosphine oxides to phosphine. Recent study has also
used it for conjugate reduction of α, β-Unsaturated Carbonyl and
Carboxyl Compounds. Finally, it is also used in greener amine
synthesis mediated by reductive amination which is an alternative to
borohydrides.
Chemische Eigenschaften
Colorless liquid
Verwenden
Polymethylhydrosiloxane(PMHS) is an easily handled,inexpensive,non-toxic,and mild reducing agent. PMHS is attractive as a substitute for more expensive or hazardous silanes or siloxanes and as the stoichiometric reductant in catalytic organotin-mediated processes.
It can be cross linked by metal catalyst at low temperature to form waterproof membrane. It is used as waterproof agent of textile, glass, pottery, paper, leather, metal, cement and marble etc, especially in textile waterproof. It is used as insulator and cross linker of paper.
Poly(methylhydrosiloxane) is used in the reduction of esters to alcohols, catalyzed by a combination of titanocene dichloride and either n-BuLi or EtMgBr. It is a safer alternative to triethoxysilane, B22063, for reduction of phosphine oxides to phosphines, catalyzed by Ti(O-i-Pr)4. It is also used in greener amine synthesis by reductive amination as an alternative to borohydrides.
synthetische
Poly(methylhydrosiloxane) is prepared by hydrolysis of methyldichlorosilane followed by heating (60–150℃) the resultant mixture of cyclic
silanes in the presence of hexamethyldisiloxane generates the
linear polysiloxane.
Allgemeine Beschreibung
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is an environmentally benign reducing agent and has been enhanced for catalytic efficiency.
Click here for more information.
Harnessing the reactivity of poly(methylhydrosiloxane) for the reduction and cyclization of biomass to high-value products
Synthese
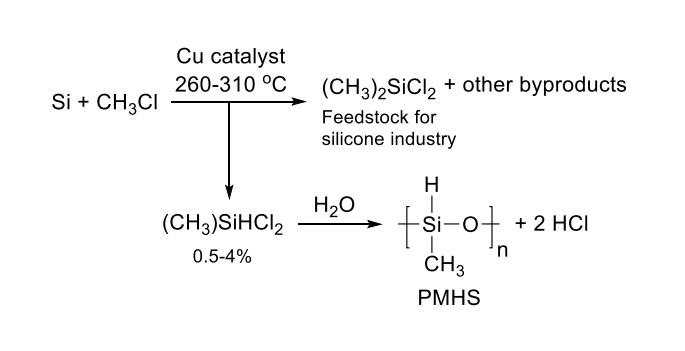
Poly(methylhydrosiloxane)(PMHS) is synthesized by the controlled hydrolysis of MeSiHCl
2[1].
Advantages
PMHS is synthesized by the controlled hydrolysis of MeSiHCl2, a byproduct in producing Me2SiCl2, a feedstock for the silicone industry. As a byproduct of the silicone industry, it is a cheap, easy to handle, and environmentally friendly reducing agent. PMHS is more air and moisture-stable than other silanes and can be stored for long periods of time without loss of activity. In addition to being synthesized from a waste product, PMHS is cheap, stable to air and moisture, and is considered nontoxic[2].
Poly(methylhydrosiloxane) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

