| Identification | More | [Name]
4-(3'-Methylphenyl)amino-3-pyridinesulfonamide | [CAS]
72811-73-5 | [Synonyms]
3-SULFONAMIDO-4-(3-METHYLANILINO)-PYRIDINE
4-(-3-METHYLPHENYL)AMINO-3-PYRIDINESULFONAMIDE
4-(3'-METHYLPHENYL)AMINO-3-PYRIDINESULFONAMIDE
SPECS AJ-333/25006115
4-(3-METHYLPHENYL)AMINO-3-PYRIDINESULFONAMIDE (SMPAP)
4-[3-Methyl phenyl] amino-3-pyridine sulphonamide
Torasemide intermediate
[4-(3-Methylphenyl)amino]pyrid
4-(3-methylphenyl)amino-3-pyridine sulfonamide (intermediate of torasemide)
4-(3-methylphenylamino) Pyridine-3-sulfonamide
[4-3-Methylphenyl)amino]pyridine-3-sulphonamide
4-(3-METHYLPHENYL)AMINO-3-PYRIDINESULFONAMIDE 98% | [EINECS(EC#)]
615-805-8 | [Molecular Formula]
C12H13N3O2S | [MDL Number]
MFCD00661332 | [Molecular Weight]
263.32 | [MOL File]
72811-73-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
162-164?C | [Boiling point ]
475.8±55.0 °C(Predicted) | [density ]
1.357±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
neat | [pka]
9.53±0.60(Predicted) | [color ]
White to Off-White | [BRN ]
5438165 | [InChIKey]
ZXPCUGWAKUIOOF-UHFFFAOYSA-N | [CAS DataBase Reference]
72811-73-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White to Off-White Solid | [Uses]
Torasemide intermediate. | [Synthesis]
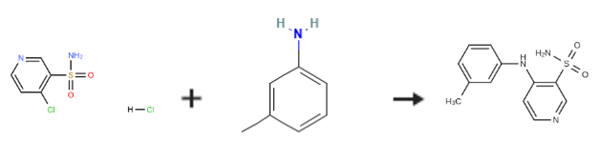
4-(3'-Methylphenyl)amino-3-pyridinesulfonamide is prepared by the reaction of 4-Chloropyridine-3-sulfonamide hydrochloride and 3-Methylaniline. The specific synthesis steps are as follows:
EXAMPLE 1; Preparation of 4-(3-methylphenyl)aminopyridine-sulfonamide; 2L three-neck flask, equipped with a mechanical stirrer, thermometer and condenser, was charged with water (500 ml) and 4-chloro-3- pyridinesulfonamide hydrochloride (100g, 0.44 mol). To this suspension was added m-toluidine (49.2 ml, 0.46 mol) at room temperature. The reaction mixture was heated to 90°C for a minimum period of 3 h. The progress of the reaction was followed by HPLC. After completion, the mixture was cooled to room temperature. The pH of the reaction was then adjusted carefully to pH 7-8 with sat. NaHC03 (ca. 1.1 l). The product was precipitated out and isolated by vacuum filtration as beige solid (126.2 g wet weight). The product was then dissolved in MeOH (1.0 l) at room temperature and charged with Darco KB (25g). The solution was refluxed for 0.5 h and then filtered through a patch of celite to remove Darco KB, while still hot, and rinsed with hot MeOH (200 ML). The filtrate was then charged with water (1.2 l) and stirred for a minimum of 1 h at room temperature. The product, which had precipitated out, was isolated by vacuum filtration to obtain a solid 106.3 g (92percent wet weight =>99.8percent purity a/a). 1H NMR (d6-DMSO) ; 2.30 (s, 3H), 7.00-7. 15 (m. 5H), 7.32 (m, 1H), 7.75 (brs, 1.5H), 8.05 (brs, 0.5H), 8.25 (d, 1H), 8.68 (s, 1H).
 |
|
|