| Identification | More | [Name]
Flutamide | [CAS]
13311-84-7 | [Synonyms]
2-METHYL-N-(4'-NITRO-3'-(TRIFLUOROMETHYL)PHENYL)PROPANAMIDE
2-METHYL-N-(4-NITRO-3-[TRIFLUOROMETHYL]PHENYL)PROPANAMIDE
AURORA KA-860
FLUTAMIDE
N1-[4-NITRO-3-(TRIFLUOROMETHYL)PHENYL]-2-METHYLPROPANAMIDE
TIMTEC-BB SBB006930
2-methyl-4’-nitro-alpha,alpha,alpha-triflouro-m-propionotoluidid
2-methyl-n-(4-nitro-3-(trifluoromethyl)phenyl)-propanamid
4’-nitro-3’-trifluoromethylisobutyranilide
alpha,alpha,alpha-trifluoro-2-methyl-4’-nitro-m-propionotoluidide
niftholide
niftolide
sch13521
2-Methyl-N-[4-nitro-3-(trifluoromethyl)-phenyl]-propionamide
Flutamide USP25
FlutamideUsp27
N-[4-Nitro-3-(trifluoromethyl)phenyl]isoButyramide
FLUTAMIDE (2-METHYL-N-(4''-NITRO-3''-(TRIFLUOROMETHYL)PHENYL)PROPANAMIDE)
Drognil
Euflex | [EINECS(EC#)]
236-341-9 | [Molecular Formula]
C11H11F3N2O3 | [MDL Number]
MFCD00072009 | [Molecular Weight]
276.21 | [MOL File]
13311-84-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Light Yellow Solid | [Melting point ]
112 °C | [Boiling point ]
400.3±45.0 °C(Predicted) | [density ]
1.3649 (estimate) | [storage temp. ]
Store at RT | [solubility ]
Practically insoluble in water, freely soluble in acetone and in ethanol (96 per cent). | [form ]
neat | [pka]
13.12±0.70(Predicted) | [color ]
Pale Yellow to Light Yellow | [Usage]
Antiandrogen; antineoplastic (hormonal). | [Merck ]
4208 | [InChI]
InChI=1S/C11H11F3N2O3/c1-6(2)10(17)15-7-3-4-9(16(18)19)8(5-7)11(12,13)14/h3-6H,1-2H3,(H,15,17) | [Contact allergens]
Flutamide is an antiandrogenic hormonal antineoplas tic drug that can induce photosensitivity and porphy ria-like eruption. | [InChIKey]
MKXKFYHWDHIYRV-UHFFFAOYSA-N | [History]
Flutamide was first described as a member of a series of N-acyl anilides synthesized at Monsanto in the 1960s during a compound finding program aiming at bacteriostatic agents. Soon after, at Schering Corp., the compound was characterized pharmacologically and further developed as SCH-13521. It was found that flutamide inhibits agonist action at the AR by replacing the agonist at the ligand binding site, being the first nonsteroidal compound possessing anti-androgenic activity in animals. In contrast to steroidal anti-androgens, for instance, cyproterone acetate, which also displays significant progestational activity, flutamide has no other hormonal activity. There is also no reduction of serum testosterone levels seen with flutamide but rather a slight increase in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) resulting in elevated serum testosterone levels. This accounts for the beneficial maintenance of libido and potency in sexually active patients. On the other hand, elevated serum estradiol levels resulting from peripheral testosterone aromatization leading to gynecomastia were observed in patients. | [SMILES]
C(NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1)(=O)C(C)C | [Uses]
Besides prostate cancer, flutamide has also been tested and/or used off-label in other hyperandrogenism-related disorders like benign prostatic hyperplasia, acne vulgaris, and hirsutism syndrome. Due to its teratogenic potential, flutamide is restricted for premenopausal women and used only in combination with effective contraception. | [CAS DataBase Reference]
13311-84-7(CAS DataBase Reference) | [EPA Substance Registry System]
Flutamide (13311-84-7) |
| Hazard Information | Back Directory | [Chemical Properties]
Light Yellow Solid | [Uses]
Antiandrogen; antineoplastic (hormonal). | [Uses]
Flutamide is a nonsteroidal antiandrogen drug; antineoplastic (hormonal). | [Uses]
neuroleptic | [Originator]
scheting (USA) | [Definition]
ChEBI: Flutamide is a monocarboxylic acid amide and a member of (trifluoromethyl)benzenes. It has a role as an androgen antagonist and an antineoplastic agent. | [Indications]
Flutamide (Eulexin) is a nonsteroidal androgen receptor
antagonist that inhibits androgen binding to its
nuclear receptor. It is effective in inducing prostatic regression
and is approved for the treatment of prostatic
carcinoma. For maximum clinical effectiveness it has to
be used in combination with a GnRH antagonist (e.g.,
leuprolide acetate) that inhibits androgen production.
Flutamide may eventually be used for the treatment of
hirsutism and male pattern baldness in women if a topical
preparation is developed. | [Indications]
Flutamide (Eulexin) is a nonsteroidal antiandrogen compound that competes with testosterone
for binding to androgen receptors. The drug is well absorbed
on oral administration. It is an active agent in
the hormonal therapy of cancer of the prostate and has
been shown to complement the pharmacological castration
produced by the gonadotropin-releasing hormone
(GnRH) agonist leuprolide. Flutamide prevents the
stimulation of tumor growth that may occur as a result
of the transient increase in testosterone secretion after
the initiation of leuprolide therapy. The most common
side effects of flutamide are those expected with androgen
blockade: hot flashes, loss of libido, and impotence.
Mild nausea and diarrhea occur in about 10% of patients. | [Indications]
Flutamide is a prodrug possessing only weak androgen antagonistic activity of its own. It is oxidized in vivo to the active principle hydroxyflutamide (6) as primary metabolite.The elimination half-life of hydroxyflutamide is relatively short, 4–6.6 h in patients after a single oral 250 mg dose of flutamide. Therefore, oral dosing of 250 mg flutamide three times daily was applied clinically. The first introduction into clinical studies was achieved in 1975 as single agent in the first-line treatment of advanced prostate carcinoma. In the United States, flutamide was finally approved by FDA in 1989 for the treatment of metastatic prostate cancer in combination with a luteinizing hormone-releasing hormone (LHRH, also referred to as gonadotropin-releasing hormone (GnRH)) agonist, for instance, leuprorelin acetate (Leuprolide(R), Lupron(R)) or goserelin acetate (Zoladex(R)).The combined androgen blockade by flutamide plus an LHRH agonist or surgical castration was introduced in order to maximize the effects of androgen ablation. Flutamide also inhibits the secretion of androgens from the adrenal gland, which is not impaired by chemical castration with LHRH agonists or by surgical castration. In addition, the AR antagonist avoids the unacceptable initial tumor flare that occurs when LHRH agonists are given alone.
Favorable response to flutamide was seen with advanced prostate carcinoma patients after single-agent treatment as well as after combination treatment. The progression of the disease was slowed and the lifetime of patients was extended. For instance, the National Cancer Institute (NCI) initiated a trial (INT-0036) and concluded that the combination of leuprolide with flutamide was more effective than leuprolide alone in patients with advanced prostate cancer. However, significant side effects were also reported.The most frequently observed adverse events are summarized in Table 1. Flutamide evidently amplifies some of the LHRH agonist-induced side effects.
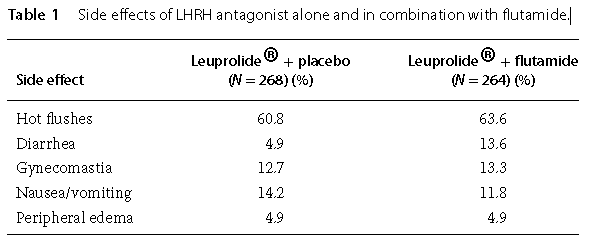
Table 1 Side effects of LHRH antagonist alone and in combination with flutamide. | [Manufacturing Process]
To a stirred, cooled solution of 100 g of 4-nitro-3-trifluoromethylaniline in 400 ml of pyridine, slowly and in a dropwise fashion, add 54 g of isobutyrylchloride and then heat the reaction mixture on a steam bath for 1.5 hours. Cool and pour the resulting mixture into ice water, filter and waterwash the crude anilide and crystallize the product of this example from benzene to obtain analytically pure material, MP 111.5°C to 112.5°C. | [Brand name]
Eulexin (Schering);DROGENIL. | [Therapeutic Function]
Antiandrogen | [General Description]
Flutamide, 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide, is dosed 3 times daily(250-mg dose; 750-mg total daily dose). A major metaboliteof flutamide, hydroxyflutamide, is a more potent AR antagonistthan the parent compound. This metabolite, which ispresent at a much higher steady-state concentration than isflutamide, contributes a significant amount of the antiandrogen action of this drug. A limiting factor in the useof flutamide is hepatotoxicity in from 1% to 5% of patients.Although the hepatotoxicity usually is reversible followingcessation of treatment, rare cases of death associated withhepatic failure have been reported to be associated with flutamidetherapy. Diarrhea is also a limiting side effect withflutamide therapy for some patients. | [Biochem/physiol Actions]
Flutamide is a non-steroidal anti-androgen drug. It consists of a nitroaromatic structure. Flutamide is a potent competitor of testosterone and dihydrotestosterone receptors. It is a potent hepatotoxin. | [Mechanism of action]
Flutamide is a nonsteroid drug that possesses antiandrogenic action. It blocks androgens
from binding with target tissues, thus preventing androgen action. The mechanism of
action is possibly also linked with a halt in dihydrotestosterone transport. It facilitates a
reduction in size and density of the prostate gland, and it reduces the amount of metastases
in such cancer, for which it is used in palliative treatment of prostate gland cancer. | [Clinical Use]
Flutamide is a pure antagonist, whereas 2-hydroxyflutamide is a more potent AR antagonist but also can activate the androgenic receptor at higher concentrations. These findings raise the possibility that increased conversion of flutamide to 2-hydroxyflutamide or accumulation of 2-hydroxyflutamide in cells may contribute to the anomalous responses to flutamide that are observed in some advanced prostate cancers. | [Synthesis]
Flutamide, 4�-nitro-3�-trifluoromethylisobutyranilide (29.2.15), a nonsteroid
antagonist of androgens, is made by acylating 4-nitro-3-trifluoromethylaniline with isobu�tyric acid chloride.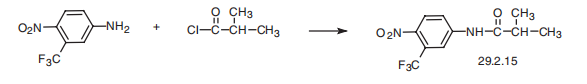
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins enhanced | [Metabolism]
It is rapidly and extensively metabolised; the
major metabolite (2-hydroxyflutamide) possesses
anti-androgenic properties. Both flutamide and
2-hydroxyflutamide are more than 90% bound to plasma
proteins.Excretion is mainly in the urine with only minor amounts
appearing in the faeces | [storage]
Store at -20°C |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R63:Possible risk of harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S27:Take off immediately all contaminated clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
UG5700000
| [HS Code ]
29242990 |
| Questions And Answer | Back Directory | [Description]
Flutamide is a kind of synthetic, non-steroidal antiandrogen which is mainly used for the treatment of prostate cancer. It is a kind of toluidine derivative and a nonsteroidal antiandrogen with a similar structure of bicalutamide and nilutamide. The mechanism of its anti-cancer property is through acting as a selective antagonist of the androgen receptor (AR), preventing androgens such as testosterone and its active metabolite dihydrotestosterone from binding to ARs in the prostate gland. This process inhibits androgen-dependent DNA and protein synthesis in the tumor cell. Therefore, it can prevent androgens from stimulating the growth of prostate cancer cells. Moreover, it can also be used for the treatment of hyperandrogenism in women. It is quite effective in alleviating symptoms such as acne, seborrhea, hirsutism and androgenetic alopecia. Finally, it is also useful as a component in the transgender hormone therapy.
| [References]
https://en.wikipedia.org/wiki/Flutamide
https://pubchem.ncbi.nlm.nih.gov/compound/flutamide#section=Top
|
|
|