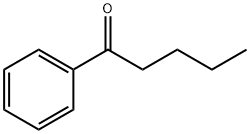
Valerophenone synthesis
- Product Name:Valerophenone
- CAS Number:1009-14-9
- Molecular formula:C11H14O
- Molecular Weight:162.23
Yield:1009-14-9 94%
Reaction Conditions:
with [2,2]bipyridinyl;palladium diacetate;trifluoroacetic acid in tetrahydrofuran;water at 80; for 36 h;Schlenk technique;Inert atmosphere;
Steps:
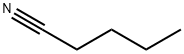
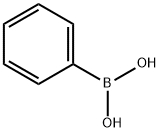
Alkyl Aryl Ketones 3 and 2-Arylbenzofurans 4; General Procedure
General procedure: To a Schlenk tube with a magnetic stirring bar were charged the respective nitrile 1 (0.4 mmol), arylboronic acid 2 (0.8 mmol), Pd(OAc)2 (5 mol%), bpy (10 mol%), TFA (10 equiv), THF (2 mL), and H2O (0.4 mL) under N2 atmosphere. The reaction mixture was stirred at 80 °C for 36 h. After cooling to r.t., the mixture was poured into EtOAc (5 mL), which was washed with sat. aq NaHCO3 (2*10 mL) and then brine (1*10 mL). After extracting the aqueous layer with EtOAc (3*10 mL), the combined organic layers were dried (Na2SO4), and evaporated under vacuum. The residue was purified by flash column chromatography (hexane-EtOAc) to afford the desired products 3 or 4. All known compounds afforded analytical data identical to those reported literature previously (see Supporting Information for further details).
References:
Wang, Xingyong;Wang, Xiaodong;Liu, Miaochang;Ding, Jinchang;Chen, Jiuxi;Wu, Huayue [Synthesis,2013,vol. 45,# 16,art. no. SS-2013-F0255-OP,p. 2241 - 2244]
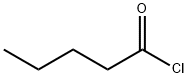
638-29-9
267 suppliers
$14.14/1gm:

71-43-2
704 suppliers
$11.19/25ML

1009-14-9
412 suppliers
$6.00/5g
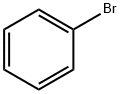
108-86-1
512 suppliers
$10.00/5g
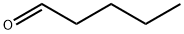
110-62-3
252 suppliers
$13.44/25ML

1009-14-9
412 suppliers
$6.00/5g
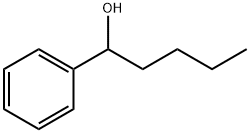
583-03-9
94 suppliers
$31.19/10g

1009-14-9
412 suppliers
$6.00/5g