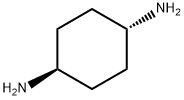
trans-1,4-Diaminocyclohexane synthesis
- Product Name:trans-1,4-Diaminocyclohexane
- CAS Number:2615-25-0
- Molecular formula:C6H14N2
- Molecular Weight:114.19
A process is disclosed for selectively making trans-1,4-Diaminocyclohexane by reacting ammonia with a mixture of cis and trans-cyclohexane-1,4-dicarboxylic acid, a lower alkyl ester, a glycol ester, an oligomeric ester or a polyester to make a solid trans-dicarboxylic acid diamide in a first step. The diamide is chlorinated to form trans-cyclohexane-1,4-dicarboxylic acid-bis-N-chloramide. The latter compound is then converted into a trans-1,4-Diaminocyclohexane with an alkali or alkaline earth metal hydroxide.
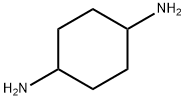
3114-70-3
207 suppliers
$12.00/1g

2615-25-0
281 suppliers
$5.00/1g

15827-56-2
78 suppliers
$28.00/100mg
Yield:30 % de
Reaction Conditions:
with 1-methoxy-2-propanol;5% rhodium on activated aluminium oxide;sodium methylate at 200; under 90009 Torr; for 2 h;Inert atmosphere;Autoclave;Pressure;Temperature;
Steps:
1 Example 1
In a 500 mL autoclave, 100 g of 1,4-diaminocyclohexane (cis / trans form = 80/20, manufactured by Tokyo Chemical Industry Co., Ltd.), 100 g of propylene glycol monomethyl ether (PGME) 7.5 g of Ru catalyst ("AA-4501", 5% Ru alumina powder, manufactured by N. E-Chemcat Co., Ltd.) and 0.63 g of sodium methoxide (manufactured by Tokyo Chemical Industry Co., Ltd.) were charged, (N 2) gas (10 kgf / cm 3) was repeated three times, and the inside of the system was replaced with nitrogen gas.While stirring at a stirring speed of 300 rpm, a temperature The reaction was carried out for 2 hours under the condition of 200 ° C. and pressure (pressure of N 2 gas) of 12 MPa. After the reaction, the reaction mixture was cooled to room temperature and pressure filtered using a 0.2 μm membrane filter to remove the catalyst to obtain a filtrate. Analysis of the obtained filtrate by gas chromatography mass spectrometry (GC-MS) revealed that the proportion of cis isomer was 63.37 area%, the proportion of trans form was 34.7 area%, the other components (4-aminocyclo By-product such as hexanol) was 1.93 area%, and cis / trans form = 65/35.
References:
JP2015/13833,2015,A Location in patent:Paragraph 0062; 0073
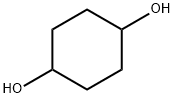
556-48-9
318 suppliers
$6.00/5g

2615-25-0
281 suppliers
$5.00/1g

15827-56-2
78 suppliers
$28.00/100mg
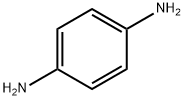
106-50-3
624 suppliers
$17.00/25g

2615-25-0
281 suppliers
$5.00/1g
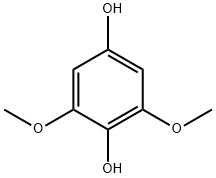
15233-65-5
89 suppliers
$45.00/100mg

2615-25-0
281 suppliers
$5.00/1g

15827-56-2
78 suppliers
$28.00/100mg
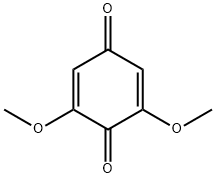
530-55-2
148 suppliers
$23.50/1g

2615-25-0
281 suppliers
$5.00/1g

15827-56-2
78 suppliers
$28.00/100mg