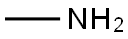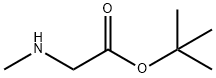
tert-Butyl sarcosinate hydrochloride synthesis
- Product Name:tert-Butyl sarcosinate hydrochloride
- CAS Number:5616-81-9
- Molecular formula:C7H15NO2
- Molecular Weight:145.2
Yield:5616-81-9 46%
Reaction Conditions:
Stage #1:methylamine with sodium iodide in acetonitrile at 0; for 0.0833333 h;
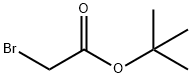
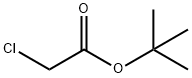
Stage #2:bromoacetic acid tert-butyl ester in acetonitrile at 20;
Steps:
4.1.10. Tert-butyl 2-methylaminoacetate (10)
To a solution of methylamine (0.80 g, 25.6 mmol) in CH3CN(3 mL) were added NaI (0.15 g, 1.02 mmol), and the suspensionwasstirred in an ice bath for 5 min. Subsequently, tert-butyl 2-bromoacetate (0.50 g, 2.56 mmol) in CH3CN (7 mL) was addeddropwise. The resulted mixture was stirred at room temperatureovernight. The solvent was evaporated under vacuum, and thewaterwas added (10 mL). Thewater phasewas extracted with ethylacetate (4 20 mL), and the combined organic phase was washedwith saturated brine and dried with anhydrous sodium sulfate.Compound 10 was obtained by filtration followed by evaporatingthe solvent without further purification. Yellow oil, 46% yield, 1HNMR (400 MHz, CDCl3) d 3.26 (s, 2H), 2.43 (s, 3H), 1.76 (s, 1H), 1.47(s, 9H). MS (ESI): m/z calcd for C7H16NO2: 146.1 [MH]; found:146.4.
References:
Zhao, Hong-Yi;Yang, Xue-Yan;Lei, Hao;Xi, Xiao-Xiao;Lu, She-Min;Zhang, Jun-Jie;Xin, Minhang;Zhang, San-Qi [European Journal of Medicinal Chemistry,2020,vol. 208,art. no. 112781]
![Glycine, N-methyl-N-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/101644-79-5.gif)
101644-79-5
0 suppliers
inquiry

5616-81-9
141 suppliers
$12.00/1g
107-97-1
588 suppliers
$5.00/100mg
540-88-5
376 suppliers
$21.00/25mL

5616-81-9
141 suppliers
$12.00/1g

107-97-1
588 suppliers
$5.00/100mg
115-11-7
241 suppliers
$45.00/25 g

5616-81-9
141 suppliers
$12.00/1g