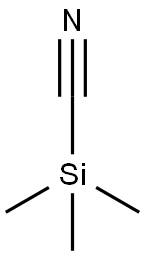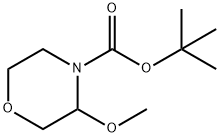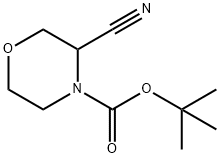
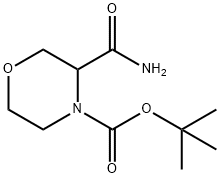
tert-butyl 3-cyanomorpholine-4-carboxylate synthesis
- Product Name:tert-butyl 3-cyanomorpholine-4-carboxylate
- CAS Number:518047-40-0
- Molecular formula:C10H16N2O3
- Molecular Weight:212.25
518047-39-7
27 suppliers
$271.00/1g

518047-40-0
61 suppliers
inquiry
Yield: 98%
Reaction Conditions:
with pyridine;oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane;acetonitrile at 0 - 20; for 0.833333 h;
Steps:
14.b
Oxalyl chloride (3.87 mL of 2M in DCM, 7.73 mmol) was added to a cooled (0°C) solution of dimethylformamide (0.598 mL, 7.73 mmol) in acetonitrile (15 mL). The solution was stirred for 20 min at 0°C. A solution of 3-carbamoyl-morpholine-4- carboxylic acid tert-butyl ester (1. 37 g, 5.95 mmol) in acetonitrile (6 mL) and pyridine (0.481 mL, 5.95 mmol) was added to the first solution. The mixture was allowed to warm to room temperature and stirred for 30 min. The solvent was removed in vacuo, and the resulting residue was dissolved in dichloromethane and washed with water. The aqueous phase was re-extracted with dichloromethane. The combined organics were dried (Na2S04- ), filtered and concentrated under reduced pressure to yield the title compound (off white crystals, 1.24 g, 98%). H NMR (300 MHz, CDCl3) 8 = 1.51 (s, 9H); 3.26 (m, 1H); 3.55 (td, J = 11.8 Hz, 2.7 Hz, 1H) ; 3.41 (dd, J = 11.8 Hz, 3.3 Hz, 1H) ; 3.83 (m, 1H) ; 3.98 (d, J = 11.4 Hz, 1H); 4.08 (d, J = 12 Hz, 1H) ; 5.32 (m, 1H).
References:
ASTRAZENECA AB;NPS PHARMACEUTICALS, INC. WO2005/80386, 2005, A1 Location in patent:Page/Page column 43-44
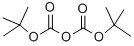
24424-99-5
863 suppliers
$13.50/25G
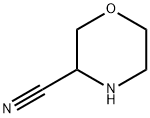
97039-63-9
29 suppliers
inquiry

518047-40-0
61 suppliers
inquiry
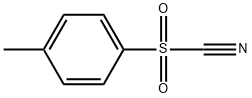
19158-51-1
178 suppliers
$52.00/1 g
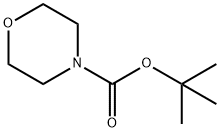
220199-85-9
72 suppliers
$10.00/5g

518047-40-0
61 suppliers
inquiry
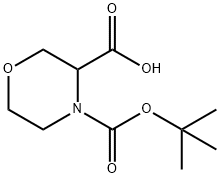
212650-43-6
159 suppliers
$46.00/250mg

518047-40-0
61 suppliers
inquiry