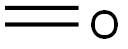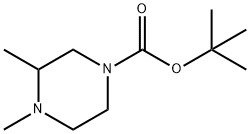
tert-Butyl 3,4-diMethylpiperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 3,4-diMethylpiperazine-1-carboxylate
- CAS Number:741287-32-1
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
Yield:741287-32-1 91%
Reaction Conditions:
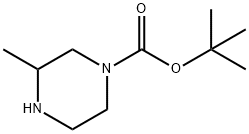
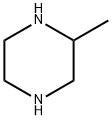
Stage #1: formaldehyd;3-methyl-piperazine-1-carboxylic acid tert-butyl esterwith sodium tris(acetoxy)borohydride in dichloromethane;water at 20; for 3 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water;
Steps:
12.2
2) 3,4-Dimethylpiperazine-1-carboxylic acid tert-butyl ester 36% Aqueous formalin (833 μL) and sodium triacetoxyborohydride (3.18 g) were added at room temperature to the above-obtained 3-methylpiperazine-1-carboxylic acid tert-butyl ester (2.0 g) in methylene chloride (40 mL), followed by stirring for 3 hours. Saturated aqueous sodium hydrogencarbonate was added thereto for partitioning the reaction mixture. The organic layer was washed with brine, and dried over magnesium sulfate anhydrate. After a filtration step, the solvent was removed under reduced pressure, to thereby give a 3,4-dimethylpiperazine compound as an oily product (1.95 g, 91%). 1H-NMR(300MHz, CDCl3) δ:1.05 (3H, d, J=6.24Hz), 1.49(9H,s), 1.96-2.05(2H,m), 2.11-2.24(1H, m), 2.28(3H,s), 2.72(1H, d, J=11.75Hz), 3.00(1H, t, J=11.20Hz), 3.81(2H,br s). MS (FAB)m/z:215 (M+H)+.
References:
EP1621537,2006,A1 Location in patent:Page/Page column 33
109-07-9
371 suppliers
$5.00/5g

741287-32-1
6 suppliers
inquiry
24424-99-5
840 suppliers
$13.50/25G

741287-32-1
6 suppliers
inquiry