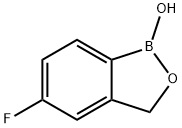
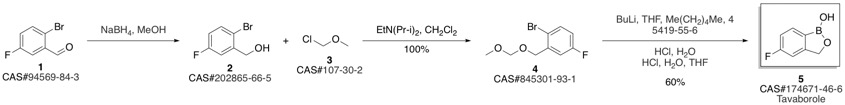
Tavaborole synthesis
- Product Name:Tavaborole
- CAS Number:174671-46-6
- Molecular formula:C7H6BFO2
- Molecular Weight:151.93
Baker, Stephen J.; Zhang, Yong-Kang; Akama, Tsutomu; Lau, Agnes; Zhou, Huchen; Hernandez, Vincent; Mao, Weimin; Alley, M. R. K.; Sanders, Virginia; Plattner, Jacob J. Discovery of a New Boron-?Containing Antifungal Agent, 5-?Fluoro-?1,?3-?dihydro-?1-?hydroxy-?2,?1-?benzoxaborole (AN2690)?, for the Potential Treatment of Onychomycosis. Journal of Medicinal Chemistry. Volume 49. Issue 15. Pages 4447-4450. Journal. (2006).
1061223-45-7
58 suppliers
$28.00/100mg

174671-46-6
217 suppliers
$7.00/10mg
Yield:174671-46-6 81%
Reaction Conditions:
with sulfuric acid at 20; for 4 h;
Steps:
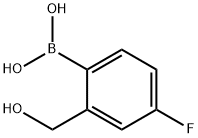
10
A mixture of crude (4-fluoro-2-(hydroxymethyl) phenyl)boronic acid (3 g) and 10% H2S04 solution (20 mL) is stirred at room temperature for 4 hrs. Water is added, and the mixture is extracted with ethyl acetate. The organic layer is washed with brine and dried on anhydrous sodium sulfate. The solvent is removed under reduced pressure, and the residue is treated with MTBE to afford benzo[c] [l,2]oxaborole-l,5(3H)-diol (Yield: 81%; Purity: 95%).
References:
BIOPHORE INDIA PHARMACEUTICALS PVT. LTD.;PULLAGURLA, Manik Reddy;NANDA KUMAR, Mecheril Valsan;PITTA, Bhaskar Reddy;RANGISETTY, Jagadeesh Babu WO2017/183043, 2017, A1 Location in patent:Page/Page column 34
![2-bromo-5-fluoro-[1-(methoxymethoxy)methyl]benzene](/CAS/20180703/GIF/845301-93-1.gif)
845301-93-1
10 suppliers
inquiry

174671-46-6
217 suppliers
$7.00/10mg
![Benzene, 1-bromo-2-[(1-ethoxyethoxy)methyl]-4-fluoro-](/CAS/20180529/GIF/651326-68-0.gif)
651326-68-0
2 suppliers
inquiry

174671-46-6
217 suppliers
$7.00/10mg

943310-52-9
0 suppliers
$36.00/100mg

7732-18-5
485 suppliers
$12.69/100ml

174671-46-6
217 suppliers
$7.00/10mg

943310-56-3
1 suppliers
inquiry

174671-46-6
217 suppliers
$7.00/10mg



