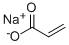
Sodium acrylate synthesis
- Product Name:Sodium acrylate
- CAS Number:7446-81-3
- Molecular formula:C3H3NaO2
- Molecular Weight:94.04
SIMONE MANZINI . Palladium- and Nickel-Catalyzed Synthesis of Sodium Acrylate from Ethylene, CO2, and Phenolate Bases: Optimization of the Catalytic System for a Potential Process[J]. European Journal of Organic Chemistry, 2015, 2015 32: Pages 7122-7130. DOI:10.1002/ejoc.201501113.
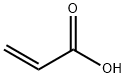
79-10-7
700 suppliers
$20.50/500ml
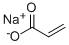
7446-81-3
190 suppliers
$5.00/1g
Yield:7446-81-3 96.8%
Reaction Conditions:
with sodium hydroxide in water;acetone;
Steps:
1 Example 1
Example 1 Preparation of Sodium Acrylate 12.01 g (0.3003 mol) sodium hydroxide and 10 ml water were added to a 50-ml Erlenmayer flask equipped with a magnetic stirring bar, and the mixture was stirred to dissolution. The sodium hydroxide solution was then carefully added to a 250-ml beaker containing 28.03 g (0.3893 mol) acrylic acid (exothermic reaction), with continuous stirring. The mixture was allowed to cool, 50 ml of acetone were added, and the precipitate vacuum filtered. The wet sodium acrylate was first air dried and subsequently dried in an oven at 55°-60° C. for 12-15 hours to obtain 27.31 g (96.8% yield) of sodium acrylate. A similar procedure was used for the preparation of potassium acrylate, except that potassium hydroxide was used instead of sodium hydroxide.
References:
US5491244,1996,A
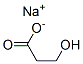
6487-38-3
22 suppliers
$39.00/100mg
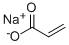
7446-81-3
190 suppliers
$5.00/1g
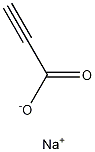
920-38-7
21 suppliers
$130.00/500mg
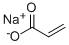
7446-81-3
190 suppliers
$5.00/1g