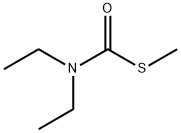
S-METHYL-N,N-DIETHYLTHIOCARBAMATE synthesis
- Product Name:S-METHYL-N,N-DIETHYLTHIOCARBAMATE
- CAS Number:37174-63-3
- Molecular formula:C6H13NOS
- Molecular Weight:147.24
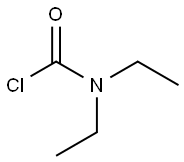
88-10-8
237 suppliers
$10.00/5g
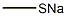
5188-07-8
285 suppliers
$13.00/5g

37174-63-3
21 suppliers
$155.00/10mg
Yield:37174-63-3 97%
Reaction Conditions:
in tetrahydrofuran;lithium hydroxide monohydrate; for 3 h;Inert atmosphere;Reflux;
Steps:
1-1 Example 1-1: /V,/V-diethyl-l-(methylsulfide)methanamide
To a solution of 200 g of 21 wt% of aqueous sodium methane thiolate solution (599 mmol) was added a solution of /V,/V-diethyl carbamoyl chloride (68.3mL, 539 mmol) in THF (600mL), followed by 4.7g (2 mol%) of tetrahexylammonium chloride. The biphasic mixture was stirred rapidly and heated to reflux under nitrogen capturing excess sulfide gasses with a bleach trap. After reflux for 3 h, the solution was cooled to rt. The biphasic reaction was extracted with 1 L of diethyl ether, and the organic phase was washed with brine and dried over sodium sulfate. After evaporation under reduced pressure, the crude oil was subjected to chromatography (Sorbtech silica gel 60; dichloromethane) to afford A,A-diethyl- 1 -(met hylsul fide) met hanamide Example 1-1 as a light yellow oil (76.8 g; 97%). NMR (400 MHz, CDCb): d 3.36 (br s, 4H, 2 x CH2), 2.30 (s, 3H, SCH3), 1.15 (br s, 6H, 2 x CH3).
References:
WO2022/115742,2022,A1 Location in patent:Page/Page column 123-124
201230-82-2
1 suppliers
inquiry

109-89-7
475 suppliers
$10.00/5g

74-88-4
351 suppliers
$15.00/10g

37174-63-3
21 suppliers
$155.00/10mg
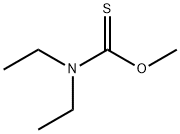
62604-36-8
4 suppliers
inquiry

37174-63-3
21 suppliers
$155.00/10mg
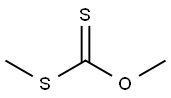
19708-81-7
8 suppliers
inquiry

109-89-7
475 suppliers
$10.00/5g

62604-36-8
4 suppliers
inquiry

37174-63-3
21 suppliers
$155.00/10mg
201230-82-2
1 suppliers
inquiry

109-89-7
475 suppliers
$10.00/5g

74-88-4
351 suppliers
$15.00/10g

37174-63-3
21 suppliers
$155.00/10mg
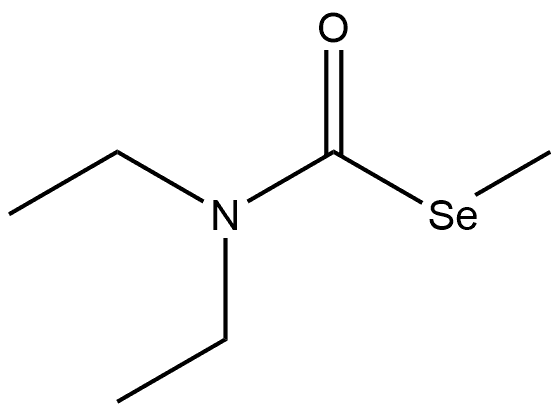
71846-97-4
0 suppliers
inquiry