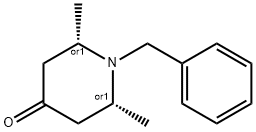
RZABKAGQMPNUFJ-TXEJJXNPSA-N synthesis
- Product Name:RZABKAGQMPNUFJ-TXEJJXNPSA-N
- CAS Number:198211-15-3
- Molecular formula:C14H19NO
- Molecular Weight:217.31
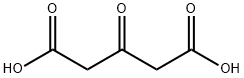
542-05-2
486 suppliers
$6.00/10g
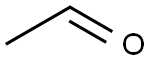
75-07-0
420 suppliers
$14.00/5mL
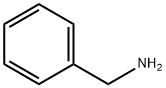
100-46-9
487 suppliers
$5.00/5G

198211-15-3
3 suppliers
inquiry
Yield:198211-15-3 19%
Reaction Conditions:
Stage #1: acetonedicarboxylic acid;acetaldehyde in lithium hydroxide monohydrate at 20; for 0.333333 h;
Stage #2: benzylamine in lithium hydroxide monohydrate at 0 - 20; for 48 h;
Steps:
12 (2S,6R)-1-Benzyl-2,6-dimethylpiperidin-4-one.
To a solution of 3-oxopentanedioic acid (100.0 g, 684.5 mmol, 1 equiv.) in water (200 mL) was added acetaldehyde (150.8 g, 1368.9 mmol, 2 equiv.) at 20° C. The reaction was stirred at 20° C. for 20 min and then cooled to 0° C. and phenylmethanamine (74.61 mL, 684.5 mmol, 1 equiv.) was added dropwise. The reaction solution was allowed to warm to room temperature and stirred for 48 h. The reaction solution was extracted with ethyl acetate 3000 mL (1000 mL×3) and the combined organic layers were washed with brine 500 mL. The organic layers were dried with anhydrous sodium sulfate, filtrated and concentrated. The crude material was purified by silica gel column chromatography to give (2S,6R)-1-benzyl-2,6-dimethylpiperidin-4-one (27.70 g, 127.5 mmol, 19% yield) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J=7.2 Hz, 2H), 7.37-7.29 (m, 2H), 7.27-7.20 (m, 1H), 3.86 (s, 2H), 3.17-3.09 (qd, J=6.4, 13.2 Hz, 2H), 2.42-2.28 (m, 4H), 1.16 (d, J=6.4 Hz, 6H)
References:
US2021/403453,2021,A1 Location in patent:Paragraph 0327-0328