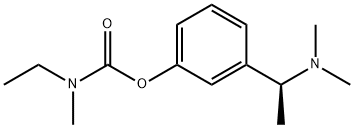
Rivastigmine synthesis
- Product Name:Rivastigmine
- CAS Number:123441-03-2
- Molecular formula:C14H22N2O2
- Molecular Weight:250.34
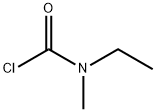
42252-34-6
285 suppliers
$7.00/1g

123441-03-2
308 suppliers
$9.00/10mg
Yield:123441-03-2 86.6%
Reaction Conditions:
Stage #1:(S)-3-(1-dimethylaminoethyl)phenol with sodium hydride in diethyl ether at 20; for 1 h;
Stage #2:N-ethyl-N-methylcarbamoyl chloride at 20; for 3 h;Reactivity;
Steps:
2 Preparation of S-(-)-rivastigmine (II)
150 ml of diethylether are placed in a 0.51-three-neck flask and sodium hydride as a 60% dispersion in oil (0.48 g) is added slowly under inert conditions (Ar or N2) and stirring. A suspension develops, to which crystalline compound (III) (2.0 g, 0.012 mol) is added at room temperature. After stirring for one hour, a slightly turbid solution of the phenolate forms, to which 1.53 g (0.012 mol) OF N-ETHYL-N-METHYLCARBAMOYLCHLORIDE in 20 ml of ether are added dropwise at room temperature. The resulting reaction mixture is stirred at room temperature for 3 hours. Thereafter, it is diluted with 100 ml of water. The organic layer is separated and extracted with 2x 50 ml of a 0.1 N NAOH solution. The organic phase is extracted with 50 ml of water, dried with anhydrous magnesium sulfate, and concentrated in vacuo. 2.6 g of an oil are obtained (86.6% of the theoretical yield).
References:
ZENTIVA, A.S. WO2004/37771, 2004, A1 Location in patent:Page 13
1412902-83-0
1 suppliers
inquiry

123441-03-2
308 suppliers
$9.00/10mg

42252-34-6
285 suppliers
$7.00/1g
![3-[(1S)-1-(Dimethylaminoethyl)]phenol](/CAS/GIF/139306-10-8.gif)
139306-10-8
316 suppliers
$9.00/250mg

123441-03-2
308 suppliers
$9.00/10mg
50-00-0
885 suppliers
$10.00/25g
![Carbamic acid, N-ethyl-N-methyl-, 3-[(1S)-1-aminoethyl]phenyl ester](/CAS/20180808/GIF/1176026-49-5.gif)
1176026-49-5
1 suppliers
inquiry

123441-03-2
308 suppliers
$9.00/10mg
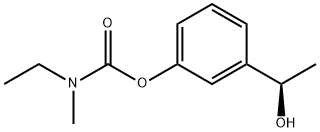
856408-80-5
6 suppliers
inquiry
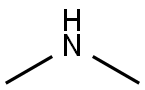
124-40-3
531 suppliers
$18.00/100ml

123441-03-2
308 suppliers
$9.00/10mg