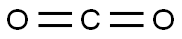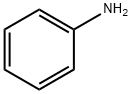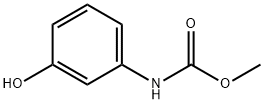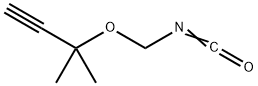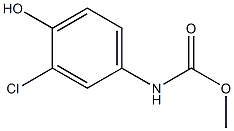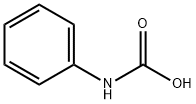
phenylcarbamic acid synthesis
- Product Name:phenylcarbamic acid
- CAS Number:501-82-6
- Molecular formula:C7H7NO2
- Molecular Weight:137.14
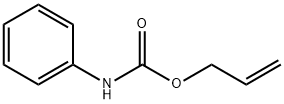
18992-89-7
2 suppliers
inquiry

501-82-6
5 suppliers
inquiry
Yield: 50%
Reaction Conditions:
with copper(l) iodide;lithium methanolate;triphenylphosphine;bis(pinacol)diborane in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
General procedure for deallylation experiments:
General procedure: To a round bottomed flask/microwave vial containing a magnetic stirrer bar was added CuI(0.1eq); PPh3 (0.13eq.); LiOMe(2eq.) and B2pin2 (1.5eq.). The flask was capped and to the vessel via syringe was added a solution of the allyl ether (1 mmol, 1 eq.) in anhydrous DMF (0.5 M). The reaction mixture was left to stir at room temperature for the stated time. After completion, the crude mixture was diluted with EtOAc and filtered through celite, washing with EtOAc. The filtrate was washed 3 times with water and once with brine then dried over magnesium sulfate and concentrated in vacuo. The crude mixture was purified by column chromatography (ethylacetate:hexanes) to give the desired deprotected compound.
References:
Hemming, David S.;Talbot, Eric P.;Steel, Patrick G. [Tetrahedron Letters,2017,vol. 58,# 1,p. 17 - 20] Location in patent:supporting information