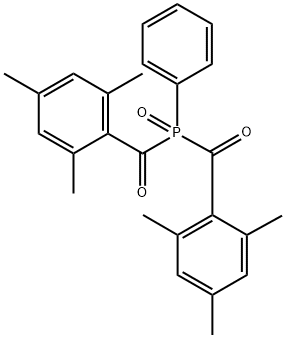
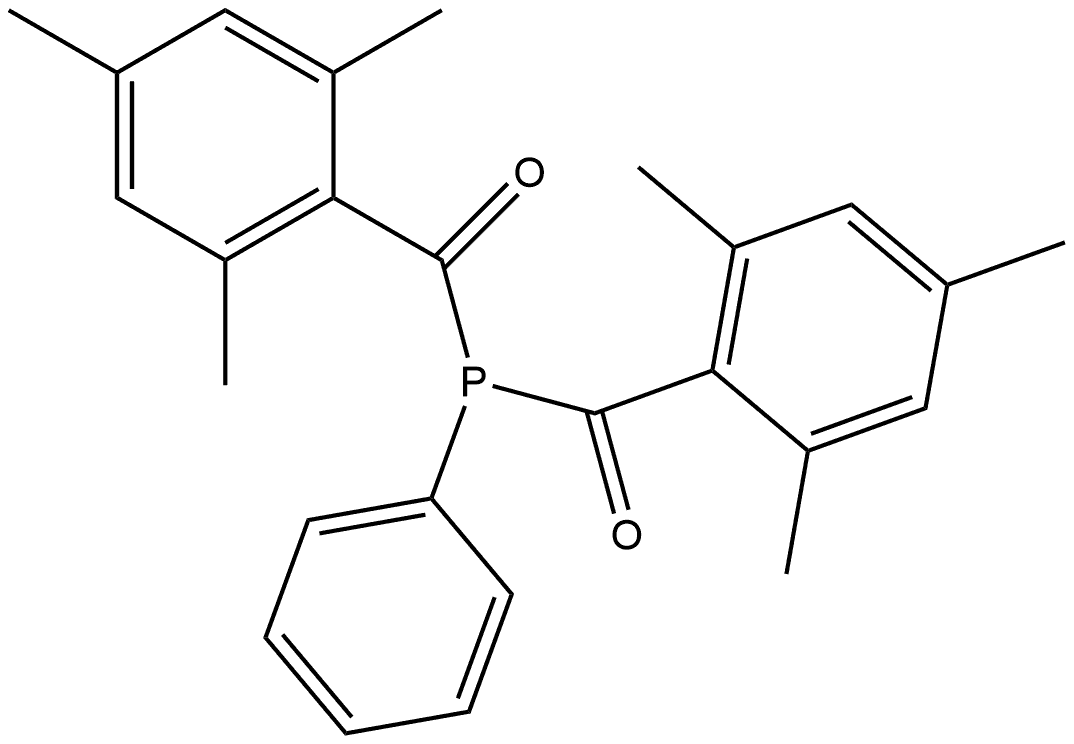
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide synthesis
- Product Name:Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
- CAS Number:162881-26-7
- Molecular formula:C26H27O3P
- Molecular Weight:418.47
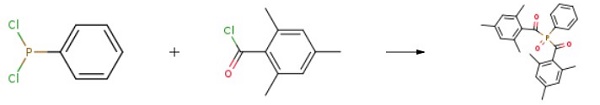
620596-61-4
0 suppliers
inquiry

162881-26-7
308 suppliers
$6.00/5g
Yield:162881-26-7 94.7%
Reaction Conditions:
with Nitrogen dioxide in toluene; for 1.5 h;Inert atmosphere;
Steps:
2
Example 2 (3) the reaction flask cooled to 50 ° C, the reaction flask to pass excess nitrogen dioxide gas, stirring the reaction solution to keep the reaction temperature constant, the intermediate product bis (2,4,6 _ - trimethyl (2, 4, 6-trimethylbenzoyl) phenyl phosphine oxide, while generating nitric oxide; passing nitric oxide gas into a gas recovery column, and oxidizing the nitric oxide gas, The tower was filled with enough air to re-oxidize nitric oxide to produce nitrogen dioxide and continue into the reaction bottle for circulation until the reaction is complete; continue stirring for 1.5 hours to complete the reaction and gas spill, stop the reaction, The excess nitrogen dioxide gas with sodium hydroxide solution absorption; (4) The reaction was stopped, separated and extracted, and the organic phase was washed twice with 250 ml of pure water, dried over anhydrous sodium sulfate, The toluene solvent was distilled off under reduced pressure to obtain a yellow target 5) The crude product was recrystallized from hexane to give a light yellow solid, which was 79.2g of the pure product of the target photoinitiator 819. The photoinitiator 819 synthesized by this method was analyzed by PLC for purity over 99.0% Phenyl phosphine dichloride, the yield was 94.7%
References:
CN104151358,2016,B Location in patent:Paragraph 0034; 0037; 0038; 0039
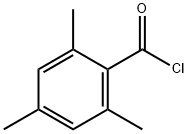
938-18-1
314 suppliers
$9.00/25g

162881-26-7
308 suppliers
$6.00/5g

824-72-6
220 suppliers
$15.00/25g

938-18-1
314 suppliers
$9.00/25g

162881-26-7
308 suppliers
$6.00/5g
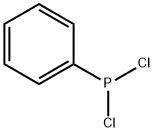
644-97-3
0 suppliers
$14.00/5g
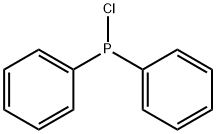
1079-66-9
448 suppliers
$5.00/5g

938-18-1
314 suppliers
$9.00/25g

162881-26-7
308 suppliers
$6.00/5g
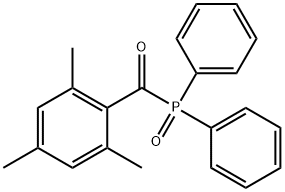
75980-60-8
490 suppliers
$14.00/5g