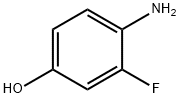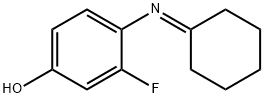
Phenol, 4-(cyclohexylideneaMino)-3-fluoro- synthesis
- Product Name:Phenol, 4-(cyclohexylideneaMino)-3-fluoro-
- CAS Number:1338722-54-5
- Molecular formula:C12H14FNO
- Molecular Weight:207.24
Yield:-
Reaction Conditions:
in cyclohexane;Reflux;
Steps:
Method 2c:A reaction flask with stirrer was charged with 41.4 g of 4-chloro-N-methyl-pyridine-2-carboxamide hydrochloride and 100 g of toluene as solvent. After addition of 68.4 g of water and 19.6 g of an aqueous sodium hydroxide solution (45 %> w/w) the reaction mixture was stirred for 30 minutes. The two phases were separated and the aqueous layer was discarded. The organic layer was concentrated by distillation under vacuum and toluene was substituted by 1 -methyl-2-pyrrolidinone (70 g) to yield a solution of 4-chloro-N-methyl-pyridine-2-carboxamide in 1 -methyl-2-pyrrolidinone.A second reaction flask with stirrer was charged with 26.7 g of 4-amino-3-fluorophenol, 73 g of cyclohexane and 20.6 g of cyclohexanone. By heating to reflux and additional stirring for 3 hours water was removed by azeotropic distillation. Then the solvent cyclohexane and the excess cyclohexanone was removed by distillation under vacuum and substituted by l-methyl-2- pyrrolidinone (70 g) to prepare a solution containing the imin compound according to the formula (III). To the resulting reaction mixture the solution of 4-chloro-N-methyl-pyridine-2-carboxamide in 1 -methyl-2-pyrrolidinone was added. The reaction mixture was heated to approximately 100°C. 126 g of potassium-t-butoxide in tetrahydrofuran (20%> w/w) was added dropwise (within approx. 40 minutes) whilst tetrahydrofuran was removed by distillation. Thereafter the reaction mixture was stirred for additional 3 hours at 100°C to complete the reaction. After adjusting to 80°C 350 ml of toluene, of 392 ml water and of 8 g acetic acid were added. The mixture was stirred for 10 minutes at 80°C, cooled down to 50°C and seeded with crystals of 4-(4-amino-3-fluorophenoxy)-N- methylpyridine-2-carboxamide. After cooling to 3°C the suspension was stirred for approximately 30 minutes. The product was filtered off, washed with methanol / water (1 :3 v/v, 144 ml) and dried under reduced pressure (30°C, 80 mbar). In this way 40.2 g (76 % of theory) of 4-(4-amino-3- fluorophenoxy)-N-methylpyridine-2-carboxamide were obtained as light brown crystals.m.p. 14PC1H-NMR (400MHz, DMSO-d6): δ [ppm]= 2.83 (d, 3H), 5.27 (s, 2H), 6.78 - 6.85 (m, 1H), 6.86 - 6.94 (m, 1H), 7.01 - 7.07 (m, 1H), 7.09 - 7.14 (m, 1H), 7.41 (d, 1H), 8.49 (d, 1H), 8.71 - 8.87 (m, 1H).MS [ES]: m/e = 262 [M+H]+HPLC: stationary phase: Agilent Zorbax SB-AQ (150 mm length, 3 mm ID, 3.5 μιη particle size); mobile phase A: 1.40 g di-potassiumhydrogenphosphat + 5.8 ml o-phosphoric acid (8.5% in water) / 1 L water; mobile phase B: acetonitrile; UV detection at 268 nm; oven temperature: 50 °C, injection volume: 3 μ, flow: 0.8 mL / min; linear gradient in two steps: 10% B -> 37% B (10 min.), 37% B - > 80% B (10 min.), 10 minutes holding time at 80% B; purity: >98% (Rt = 9.1 min.).
References:
WO2011/128261,2011,A1 Location in patent:Page/Page column 16