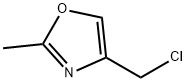
OXAZOLE, 4-(CHLOROMETHYL)-2-METHYL- synthesis
- Product Name:OXAZOLE, 4-(CHLOROMETHYL)-2-METHYL-
- CAS Number:141399-53-3
- Molecular formula:C5H6ClNO
- Molecular Weight:131.56
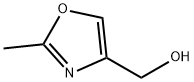
141567-53-5
71 suppliers
$90.00/100mg

141399-53-3
27 suppliers
$200.00/100mg
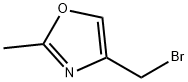
657389-99-6
12 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:4-hydroxymethyl-2-methyloxazole with methanesulfonyl chloride;triethylamine in N,N-dimethyl-formamide at 0; for 1 h;Inert atmosphere;
Stage #2: with lithium bromide in N,N-dimethyl-formamide at 0; for 1 h;
Steps:
36.1.A Mixture of: 4-(bromomethyl)-2-methyl-1,3-oxazole and 4-(chloromethyl)-2-methyl-1,3-oxazole
Under argon, 1.00 g (8.84 mmol) of (2-methyl-1,3-oxazol-4-yl)methanol and 1.60 ml (11.49 mmol, 1.3 eq.) of triethylamine were dissolved in 12.5 ml of DMF and cooled to 0° C. At this temperature, 0.890 ml (11.49 mmol, 1.3 eq.) of methanesulphonyl chloride was added dropwise and the mixture was stirred at 0° C. for 1 h. 2.15 g (24.75 mmol, 2.8 eq.) of lithium bromide were then added, and the mixture was stirred at 0° C. for 1 h. The reaction mixture was admixed with water and extracted three times with ethyl acetate. The combined organic phases were washed with saturated aqueous sodium chloride solution, dried over sodium sulphate and concentrated. The residue was purified by means of Biotage-Isolera (eluent: dichloromethane). Yield: 3.00 g (38% purity, 73% of theory). (0960) LC/MS [Method 9]: Rt=2.73 min; MS (ESIpos): m/z=177 (M+H)+, (0961) LC/MS [Method 9]: Rt=2.19 min; MS (ESIpos): m/z=133 (M+H)+.
References:
BAYER PHARMA AKTIENGESELLSCHAFT;R?HRIG, Susanne;JIMENEZ NUNEZ, Eloisa;SCHLEMMER, Karl-Heinz;TERSTEEGEN, Adrian;TELLER, Henrik;HILLISCH, Alexander;HEITMEIER, Stefan;SCHMIDT, Martina Victoria;STAMPFU?, Jan US2017/298052, 2017, A1 Location in patent:Paragraph 0958-0961
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

141399-53-3
27 suppliers
$200.00/100mg
60-35-5
507 suppliers
$10.80/1g
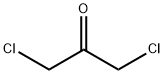
534-07-6
9 suppliers
$10.00/1g

141399-53-3
27 suppliers
$200.00/100mg