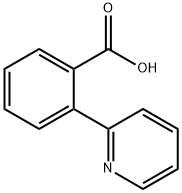
OTAVA-BB 1364602 synthesis
- Product Name:OTAVA-BB 1364602
- CAS Number:13764-20-0
- Molecular formula:C12H9NO2
- Molecular Weight:199.21
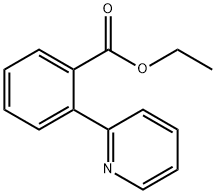
28901-52-2
4 suppliers
inquiry

13764-20-0
44 suppliers
$96.00/100mg
Yield:13764-20-0 89%
Reaction Conditions:
with sodium hydroxide in ethanol;Inert atmosphere;Reflux;Darkness;
Steps:
4.3.29. General procedure - Ester hydrolysis
General procedure: A solution of the biaryl ester 21-31 (1 eq) and NaOH (2.1 eq) inethanol (2 mL) was stirred at reflux overnight, darkening over time. Thefollowing morning the reaction was allowed to cool and carried through one of three work-up methods: Method A: The mixture was quenched with water (5 mL) and thevolatile components of the mixture were removed in vacuo. Theremaining solution was washed with chloroform (5 mL), acidified to pH4 - 5 using 2 M aqueous HCl and extracted with a 3:1 mixture of chloroformand isopropanol (3 × 5 mL). The combined extract was driedover anhydrous MgSO4 and the solvent was removed in vacuo to yieldthe desired acid.
References:
Gallagher, Ryan;Qudah, Taima;Balle, Thomas;Chebib, Mary;McLeod, Malcolm D. [Bioorganic and Medicinal Chemistry,2021,vol. 51,art. no. 116516]
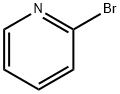
109-04-6
532 suppliers
$6.00/25g
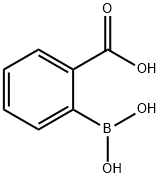
149105-19-1
290 suppliers
$17.00/1g

13764-20-0
44 suppliers
$96.00/100mg
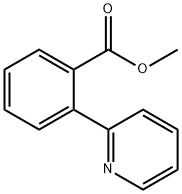
98061-19-9
2 suppliers
inquiry

13764-20-0
44 suppliers
$96.00/100mg
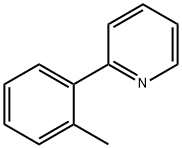
10273-89-9
40 suppliers
$355.25/1g

13764-20-0
44 suppliers
$96.00/100mg
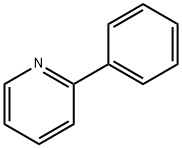
1008-89-5
282 suppliers
$7.00/5g

13764-20-0
44 suppliers
$96.00/100mg