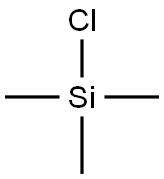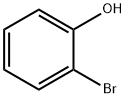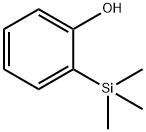
o-(Trimethylsilyl)phenol synthesis
- Product Name:o-(Trimethylsilyl)phenol
- CAS Number:15288-53-6
- Molecular formula:C9H14OSi
- Molecular Weight:166.29
![[(2-Bromophenyl)oxy]trimethylsilane](/CAS/GIF/36601-47-5.gif)
36601-47-5
3 suppliers
inquiry

15288-53-6
37 suppliers
inquiry
Yield: 93%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran at -78 - 20; for 4 h;Inert atmosphere;
Steps:
4.10.2 Synthesis of 2-(trimethylsilyl)phenol [42]
2-Bromophenoxy) trimethylsilane, 10g, 41mmol, 1 eq.) was dissolved in dry THF (50mL) and cooled to-78°C under nitrogen with continuous stirring. To this solution dropwise of n-BuLi (38.5mL of 1.6M in hexane, 61.5mmol, 1.5 eq.) was added by syringe. The reaction mixture was stirred for three hours in same temperature, then warmed to rt and stirred for one hour. The reaction mixture quenched by adding saturated ammonium chloride (10mL). The reaction mixture was allowed to room temperature and then extracted it with ethyl acetate (3×25mL). The organic phase was washed with water, brine, and dried over MgSO4 and the reaction mixture taken to dryness in vacuo. The obtained light brown oil was purified by flash column chromatography (silica gel, hexane-EtOAc, 10:1) to give the title compound 2-(trimethylsilyl)phenol as colourless oil. Yield 6.31g (93%). v v max/cm-1(ATR): 3600-3300 (broad, O-H str.), 3080 (=C-H str.), 2959 (-C-H str.), 1593 (C=C str.), 1436, 1241, 1122, 1072; 1H NMR (400MHz, CDCl3): δ=7.31 (d, J=7.0, 1H), 7.11-7.17 (m, 1H), 6.86 1H, tt, J=7.0, 1.0Hz,1H), 6.51 (1H, d, J=8.0Hz, Ar-H6), 4.92 (1H, s, broad, OH), 0.26 (s, 9H, CH3); 13CNMR (100MHz, CDCl3): δ=161.3 (C2), 136.2 (C6), 131.6 (C4), 126.4 (C1), 121.6 (C5), 115.4 (C3). LRMS (EI) C9H14OSi requires 166.0863; found (EI) 166.0864 [M+.], (Δ=0.87ppm).
References:
Rashid, Srood O.;Almadhhi, Sultan S.;Berrisford, David J.;Raftery, James;Vitorica-Yrezabal, Inigo;Whitehead, George;Quayle, Peter [Tetrahedron,2019,vol. 75,# 16,p. 2413 - 2430]
18036-83-4
8 suppliers
inquiry

15288-53-6
37 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

15288-53-6
37 suppliers
inquiry