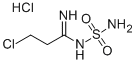
N-Sulphamyl-3-chloropropionamidine hydrochloride synthesis
- Product Name:N-Sulphamyl-3-chloropropionamidine hydrochloride
- CAS Number:106649-95-0
- Molecular formula:C3H9Cl2N3O2S
- Molecular Weight:222.09
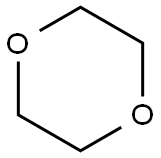
123-91-1
684 suppliers
$16.00/25g
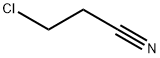
542-76-7
145 suppliers
$31.00/25mL

106649-95-0
128 suppliers
inquiry
Yield:106649-95-0 86.2%
Reaction Conditions:
with hydrogenchloride;SULFAMIDE in hydrochloric acid 1,4-dioxane
Steps:
3 N-sulfamyl-3-chloro-propionamidine hydrochloride
Example 3 N-sulfamyl-3-chloro-propionamidine hydrochloride Sulfamide (13.46 g; 14.0 cmoles) was added to a solution of 3-chloropropionitrile (41.8 ml; 53.2 cmoles) in 1,4-dioxane hydrochloride (114.0 g, 30% w/w). The mixture was heated under stirring to a temperature of 55°-60°C for four hours at a pressure of 1.0-1.5 kp/cm, which was held by the addition of more hydrogen chloride. A slightly cloudy solution resulted. The excess acid was removed, and collected over 1,4-dioxane (100 ml), cold, the mixture being exhausted by heating to 80°-85°C. The removal of the hydrogen chloride remains was completed by evaporation at reduced pressure. The resulting solution was cooled in a water-ice bath (0°-5°C) and diluted with dichloromethane (100 ml) to give an oil, which was solidified by stirring at room temperature over a period of time (30 min.). It was cooled to 0°-5°C again for 45 min., filtered, washed with dioxane (twice, 30 ml) and with dichloromethane (twice, 40 ml). After drying, it gave the title compound (26,8 g), yield 86.2% of theory, m.p.: (136°C, with softening) 139°-142°C and I.R. (KBr), νcmmin1: 3340, 3300, 3000, 2480, 1680, 1630, 1505, 1170, 920 and 815 (most representative readings). Hydrogen chloride recovered, 20.0 g.
References:
CENTRO MARGA PARA LA INVESTIGACION S.A. EP356366, 1990, A1

60-29-7
7 suppliers
$30.00/50g

106649-95-0
128 suppliers
inquiry

107-13-1
413 suppliers
$16.00/25ML

106649-95-0
128 suppliers
inquiry
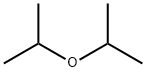
108-20-3
564 suppliers
$25.00/25mL

107-13-1
413 suppliers
$16.00/25ML

106649-95-0
128 suppliers
inquiry