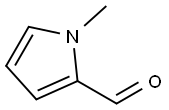
N-Methylpyrrole-2-carboxaldehyde synthesis
- Product Name:N-Methylpyrrole-2-carboxaldehyde
- CAS Number:1192-58-1
- Molecular formula:C6H7NO
- Molecular Weight:109.13
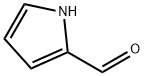
1003-29-8
452 suppliers
$10.00/1g
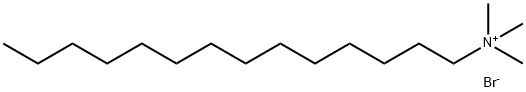
1119-97-7
495 suppliers
$5.00/25g

1192-58-1
181 suppliers
$9.00/5g
Yield:1192-58-1 46%
Reaction Conditions:
with sodium hydroxide in water;toluene for 5 h;Reflux;chemoselective reaction;
Steps:
4.2. General Procedure for the Synthesis of 1b-7b and 11b.
General procedure: A 100mL round-bottom flask was equipped with a magnetic stir bar and a reflux condenser. To xylene (10.0mL), tetradecyltrimethylammonium bromide (1.1mmol) and a heterocyclic compound (1.0mmol) were added, followed bya solution of NaOH 50% (5.0 mL). The mixture was stirred at reflux temperature for 2-18 h. After completion of thereaction, the mixture was air-jet cooled to 25 °C and TLC indicated the disappearance of the starting material. The reaction mix was treated with AcOEt (4 × 20 mL), and the organic phase separated and removed under reduced pressure. The residue was purified to analytical purity by column chromatography.
References:
González-González, Carlos A.;Vega, Juan Javier Mejía;Monroy, Ricardo García;González-Calderón, Davir;Corona-Becerril, David;Fuentes-Benítes, Aydeé;Mascarúa, Joaquín Tamariz;González-Romero, Carlos [Journal of Chemistry,2017,vol. 2017,art. no. 4586463]
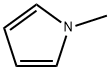
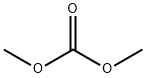
96-54-8
281 suppliers
$5.00/1g

1192-58-1
181 suppliers
$9.00/5g
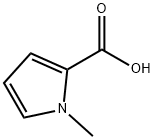
6973-60-0
204 suppliers
$6.00/250mg

1192-58-1
181 suppliers
$9.00/5g